General Information/Reaction mechanisms
There are a limited number of transposase types defined by their chemistry.
Contents
The main groups
Two groups whose mechanisms are the best understood are the DDE and the HUH superfamilies named after the amino acids which constitute the active sites. The reaction mechanisms involved in transposition using DDE enzymes have been treated in depth (e.g. [1][2][3][4] as have those of the HUH enzymes (see [5][6]).
Other enzymes
Other types of transposase include: the DEDD enzymes[7] whose chemistry presumably resembles that of the DDE enzymes (since they also have an RNaseH fold[8]) but resemble the RuvC four-way Holliday junction resolvase; Serine transposases which are thought to use chemistry similar to their Serine-site-specific recombinase cousins; and a group of novel transposons, casposons, thought to be primitive ancestors of the CRISPR system[9].
The transpososome
Most transposition reactions can be divided into several defined steps generally comprising: binding of the transposase to the ends; elaboration of a synaptic complex involving the transposase, perhaps accessory proteins, and both transposon ends - this step involves either concomitant or subsequent (depending on the element) recruitment of the target DNA; cleavage and strand transfer of the transposon ends into the target; and processing of the strand transfer complex to a final product. The protein-DNA complexes assembled during this process are called transpososomes.
Enzymes or reaction components?
An important point which has not yet been fully addressed is whether transposases are true enzymes which can be recycled or whether they are themselves reaction components which are consumed during the process. The fact that many transposases show a preference for action on the element from which they are expressed (in cis) and the coupling between transposase translation and DNA binding[10] (10) imposes strong constraints on transposase recycling. Moreover, at least in the case of bacteriophage Mu, special machinery in place for removing transposase from the transposition complex following insertion which includes ClpX[11][12][13]. In addition, the Lon protease is implicated in proteolysis of the IS903 transposase[14][15].
Chemistry of the DDE enzymes
The DDE catalytic site
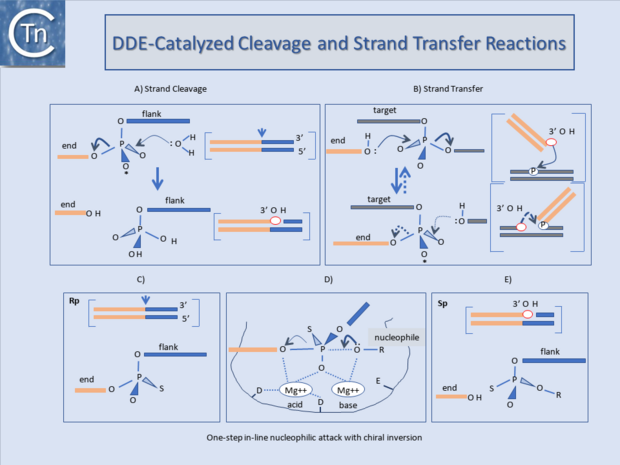
It has become clear that many of the enzymes involved in transposition reactions are related and, moreover, are part of a larger family of phosphoryltransferases which also includes RNaseH and the RuvC "Holliday junction resolvase" [16][17][18][19][20][21][22][4].
These transposases catalyse cleavage at the 3' ends of the element by an attacking nucleophile (generally H2O) to expose a free 3'OH group (Fig.23.1). This hydroxyl in turn acts as a nucleophile to attack a 5' phosphate group in the target DNA (strand transfer) in a single-step transesterification reaction. Under certain conditions the enzyme is also capable of "disintegrating" the transposon end by catalysing the attack of the 3' target OH group on the new transposon-target junction[23][24][25].
The reaction(s) do not require an external energy source and do not involve a covalently linked enzyme-substrate intermediate as do certain site-specific recombination reactions. It is worth underlining that, since it is the donor strand itself which performs the cleavage-ligation step in the target DNA, no cleaved target molecule is detected in the absence of strand transfer.
An acidic amino acid triad (DDE : Asp, Asp, Glu) present in these enzymes is intimately involved in catalysis presumably by co-ordinating divalent metal cations (in particular Mg2+) which assist the various nucleophilic attacking- and leaving-groups during the course of the reaction (Fig.23.1) The reaction is an in-line nucleophilic attack resulting in chiral inversion of the target phosphate. Chiral inversion has been observed for retroviral integration[26][27], bacteriophage Mu[28], and Tn10[29] transposition and in a related reaction, V(D)J recombination[30] and was revealed by substituting a non-bridging oxygen for a sulphur group which fixes the normally achiral phosphate group in one or other of its alternative chiral forms (Fig.23.1). For many ISs and the retroviral integrases (IN) this triad is known as the DD(35)E motif and is highly conserved[31][32][33][34]. In addition, alignments of several Tpases[35] revealed regions of amino acid conservation designated, N1, N2, and N3 and C1[35] which encompass the D (N2), D (N3) and E (C1) regions of typical DDE motifs respectively. The C1 region is probably the most defined structural element. It appears to be part of an a-helix with additional conserved amino acids including a K or R residue approximately 7 amino acids or two helical turns downstream from the E residue[36][37][38]. Less well-conserved residues often occur at approximately one helical turn (3 or 4 residues) upstream and downstream E (DDE motifs). For retroviruses this has been shown to interact with the terminal base pairs of the element presumably contributing to correct positioning of the transposon end in the active site [37][39].
Although this conservation in the primary sequence is lower in certain of the other groups of elements and not all families have been explored in sufficient detail to assure that the alignments are biologically relevant, mutagenesis studies with some of these elements (e.g. the Mu, Tn7, IS10, and Tc1/3 Tpases and the retroviral integrases) clearly underline the importance of these residues. Moreover, structural analysis showed that these acidic amino acids are arranged close to each other in a similar, three-dimensional manner in other phosphoryltransferases such as RNaseH and RuvC [16][17], otherwise unrelated to Tpases. These primary amino acid conservations are also reflected in conserved structural features. Major conserved features identified in the structures of the catalytic cores of retroviral integrases (Fig.23.1), and the Mu and IS50 Tpases include 2 beta-sheets each harboring one of the D residues and the long beta-helix including the E residue. The alfa-helix is designated α-4 in HIV and C1 in the Tpases[35][40], see [41]). It is one of the most conserved regions in the catalytic core. Mutagenesis and crosslinking studies with INHIV suggested that it played a role in positioning both the nucleophile and viral DNA[37][39][42]. In particular K159 or K156, which cross-link to the terminal CA dinucleotide, are located on the same side of the alfa-helix and are strongly conserved. Q148 also lies on the same side and appears to interact with the terminal end of the non-processed strand (see [43]).
An amide or basic amino acid is highly conserved at this or the neighboring position and mutation results in severe impairment of catalysis (IN: [42]; IS10: [44]; IS50: see [45]; IS903: [46].
Transposition reactions and different types of gene rearrangement.
If DDE transposases are capable of catalysing only single strand cleavage to generate a 3’OH at the end of the transposon, how do IS elements with move from one place to another While initiation of a transposition reaction catalysed by the DDE transposases proceeds via transfer of the 3' end of the transposon (transferred strand), the outcome of the reaction is governed by cleavage of the 5' (non-transferred) end of the element as discussed in Groups with DDE Transposases (Fig.7.4).
Cleavage of the transferred strand alone
5' strand cleavage does not occur concomitantly with 3' strand transfer (Fig.7.4), the donor and target molecules become covalently linked. Subsequent 5' strand cleavage will separate the element from the donor backbone and will also result in direct insertion (Fig.7.4). In the case of retroviruses, only the 3' cleavage occurs, removing 2 bp from the end of the double strand DNA viral copy. However, since no donor backbone is attached to the viral DNA, direct insertion can ensue. In cases where the 5’ transposon end remains attached to the donor backbone DNA the result of 3' strand transfer is to join transposon and target leaving a 3'OH in the target DNA at the junction. This can act as a primer for replication of the element and generate cointegrates where donor and target molecules are separated by a single transposon copy at each junction. During its lytic cycle bacteriophage Mu similarly undergoes only 3' cleavage of the transferred strand, the donor backbone remains attached and cointegrate molecules result if replication occurs. Members of the Tn3 family appear to transpose in this way and certain Tn7 and IS903 mutants can be induced to undergo cointegrate formation.
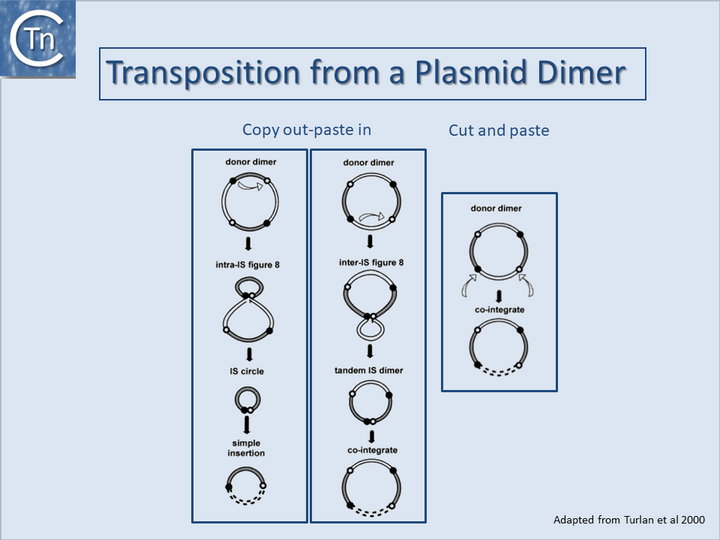
Cointegrates from donor dimer plasmids
It is important to note that cointegrates identical to those produced by replicative transposition can also be produced by a non-replicative process either from a plasmid dimer[47][48][47] (Fig.23.2) or from tandemly repeated copies of an IS element[49][50][51][52][53][54]. For copy out – paste in transposition, the two outcomes lead to either simple insertion (Fig.23.2, left) or cointegrates (Fig.23.2, middle). For cut and paste transposition, a dimer plasmid donor gives rise directly to a cointegrate structure (Fig.23.2, right).
Transposase structures and the Transpososome
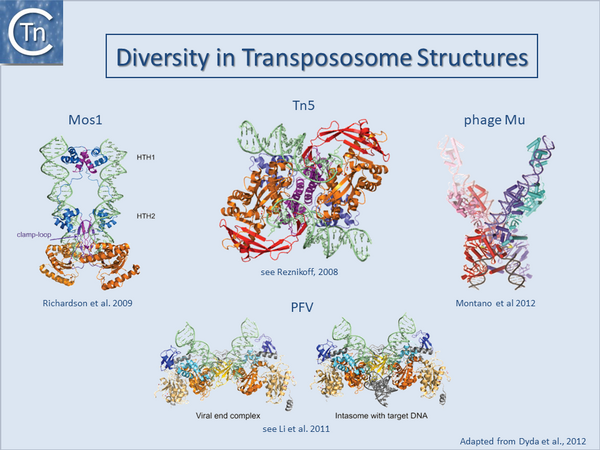
Only a single prokaryotic IS DDE transposase, that of IS50, has yielded structural information[55][56] and this has provided important insights into transposition of its cognate IS. The only other prokaryotic DDE whose structure is available is that of the transpososome of bacteriophage Mu[19]. On the other hand, a limited number of transposase and DDE transposase-DNA co-complex structures are available and have yielded important insights into the details of the transposition reaction (Fig.23.3) and (Fig.7.3).
They all tend to support the detailed reaction mechanism. These include several retroviral integrases (IN) such as HIV, ASV and PFV[43][57][58][59][60][61][62][63]; the mariner transposon Mos1[64][65][66][67]; and the hAT transposon HERMES (revealing a complex set of interactions between neighboring monomers in the octomeric transposase complex[68][69][70].
These studies underline a large degree of diversity in the different transposase/integrase and in their architecture and the detailed interaction with their cognate DNA sequences even though they share a well conserved catalytic domain topology. A recurring theme in all these structures is that the complexes tend to be stabilised by a network of inter- and intra-protein contacts and contacts with the DNA. Moreover, the cleavages appear to occur “in trans”: cleavages on one end are catalysed by a transposase molecule bound to the other DNA end [18][21][22].
This phenomenon includes both prokaryotic transposable elements Tn5[71] and Mu[72][73] as well as the eukaryotic Mos1 transposon[74] and retroviruses[75][76]. This arrangement represents an important regulatory mechanism since no chemistry can occur on a single isolated transposon end which would result in non-productive transposition and would have a negative impact on the host replicon.
Cleavage can only be initiated in the context of an architecturally defined and properly assembled “transpososome” in which both DNA ends are synapsed and which can give rise to productive transposition.
Chemistry of HUH enzymes
Mechanism and Overall Protein Architecture
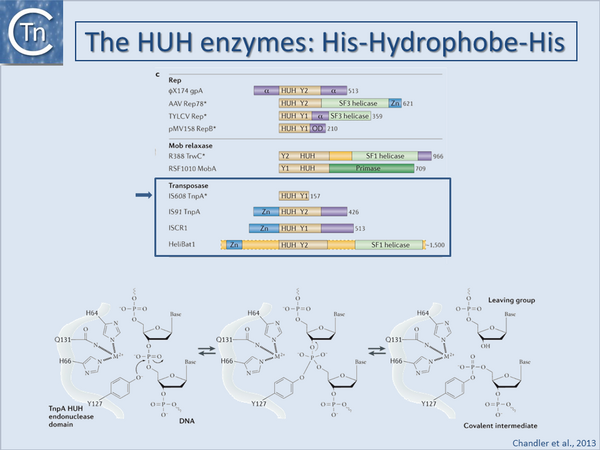
Historically, bacteriophage Φ-X174 protein A (gpA) was the first identified HUH superfamily member although, surprisingly, no structural information is available. Many related proteins subsequently identified by bioinformatics[77][78] included proteins involved in catalysis of viral and plasmid Rolling Circle Replication (RCR), conjugative plasmid transfer, and DNA transposition[79][80][81][82][83]. They all carry conserved protein motifs, including the "HUH" motif composed of two histidine (H) residues separated by a bulky hydrophobic (U) residue, and the Y-motif containing either one or two tyrosine (Tyr) residues (found in Y1 and Y2 enzymes respectively).
Y1 HUH enzymes (Fig.23.4) include Rep proteins of some plasmids with ssDNA replication intermediates (such as pUB110[84]), a wide range of eukaryotic viruses[85], most conjugative plasmid relaxases[86][87], ISCR (insertion sequences related to IS91 [82]) and IS200/IS605 insertion sequence family transposases [80][81]. Y2 enzymes include ΦX174 gpA itself, Rep proteins of other isometric ssDNA and dsDNA phages (e.g. phage P2[88]), some cyanobacterial and archaeal plasmids and parvoviruses (e.g. adeno-associated virus, AAV) as well as transposases of the IS91 and helitron families[79], and MOBF family plasmid relaxases. In some cases, both Y residues are mechanistically important while for others, only one of the pair appears to be essential.
HUH enzymes use a unique mechanism for catalysing ssDNA breakage and joining. The active site tyrosine creates a 5'-phosphotyrosine intermediate and a free 3'-OH at the cleavage site (Fig.23.4). The 3'-OH can be used for different tasks. The most obvious is to prime replication, as observed for HUH domains in single-stranded phage Rep proteins, RCR plasmids and conjugative relaxases. The 3'-OH group can also act as the nucleophile for strand transfer to resolve the phosphotyrosine intermediate in the termination step of RCR replication, conjugative transfer and transposition. The HUH enzyme cleavage polarity is inverse to that of the tyrosine recombinases, which make 3' phosphotyrosine intermediates and generate free 5'-OH groups that cannot be used as replication primers[89]. HUH enzymes also require a divalent metal ion to facilitate cleavage by localizing and polarising the scissile phosphodiester bond in contrast to the cofactor-independent tyrosine recombinases. Depending on the enzyme, Mg2+, Mn2+ or other divalent metal ions can be used in vitro[90][91][92][93][94][95]. It is presumed that Mg2+ or Mn2+ are the physiological cofactors. The HUH histidine pair provides two of the three ligands necessary for metal ion coordination (Fig.23.4). The location and identity of the third, invariably polar (Glu, Asp, His or Gln) residue varies across the superfamily.
3D structures of several Rep and relaxase HUH domains with and without bound DNA are available [e.g. [91][92][93][96][97][98][99]]. The order of HUH and Y motifs varies in the primary sequence: in the Relaxase group, the Y-motif is upstream of the HUH-motif whereas in the Rep group it is downstream (Fig.23.4). This “circular permutation” [78][98][100] changes the domain topology. Nevertheless, the three-dimensional constellation of active site residues is virtually identical across the superfamily.
Given the diverse HUH protein functions, it is not surprising that other domains are often appended to the HUH domain (Fig.23.4). These are often of unknown function but, ATP dependent helicase, zinc binding, primase and multimerisation domains are recurring themes. For example, the ssDNA substrates needed by HUH enzymes can be generated by a dedicated DNA helicase domain C-terminal to the HUH domain [79][101][102][103] or alternatively by recruitment of a host helicase. RCR processes use 3´-5´helicase activity acting on the template strand to facilitate DNA unwinding at the replication fork while in conjugation, helicases (as part of the relaxase) are transported into the recipient cell and track 5´to 3´on the transported ssDNA.
DNA Recognition
Many HUH nucleases recognize and bind DNA hairpin structures with cleavage sites located within the hairpin or in the ssDNA on the 5' or 3' side of the stem. The crucial role of hairpins has been firmly established in many systems including plasmid conjugation, eukaryotic viral and plasmid replication and transposition[80][81][91][96][104][105][106]. In other systems, palindromic sequences that can form DNA hairpins are present near the probable HUH nuclease cleavage sites [107]. Such hairpins can be formed in vivo under a number of physiological conditions (see [108]).
Structural studies revealed that small DNA hairpins can be recognized in several different ways: sequence-specific recognition of the dsDNA stem; structure-specific recognition of irregularities in the stem; or sequence-specific recognition of the hairpin loop[81][108][95][96][97][98].
The hairpin-flanking DNA - in many cases in single-stranded form - is also often important for recognition. Relaxases, for example, make extensive contacts with the bases extending between the hairpin and cleavage site[95][98][99], and for a relative of IS200/IS605 transposases, TnpAREP, nucleotides on the 5' side of the hairpin are crucial for binding and sequence-specific recognition[96]. Other family members [97][109] [23,39], have more complex binding modes.
HUH enzymes as transposases
Transposases of members of the IS200/IS605 [80], IS91[110] and ISCR [82] insertion sequence families and the eukaryotic helitrons [79] are also HUH enzymes. Those of the IS200/IS605 family are the best understood.
IS200/IS605 family
IS200/IS605 family transposases are single domain proteins with only the essential HUH motif and a single catalytic Tyr (Y1 transposases, (Fig.23.4). Both TnpAIS608 and TnpAISDra2 are obligatory dimers and the active sites are believed to adopt two functionally important conformations, one in which each is composed of the HUH motif from one monomer and the Tyr residue carried by an alpha-helix (αD) from the other (trans configuration), and the other in which both motifs are contributed by the same monomer (cis configuration). Only the former has been observed crystallographically. Similar proteins are sometimes found associated with Repeated Extragenic Palindromes (REP sequences whose hairpin structures resemble the ends of IS200/IS605 family members.
RCR transposons: IS91, ISCR and Helitron families
The earliest identified HUH domain transposases were those of the IS91 family and are significantly larger than Y1 transposases (Fig.23.4), carry a Y2 motif and include an N-terminal zinc binding motif and additional domains of yet unidentified function. A group of related elements, the ISCRs often associated with a variety of antibiotic resistance genes (see [82]) carry an orf (the CR or common region) resembling IS91 family transposases but with only a single Tyr (Fig.23.4). In addition, eukaryotic relatives, the Helitrons, have been identified by bioinformatic approaches.
Chemistry of DEDD enzymes
DEDD enzymes are limited to transposases of the IS110 family at present[111] and closely related to the Piv and MooV invertases from Moraxella lacunata / M. bovis[112][113] and N. gonorrhoeae[114][115] respectively. Piv catalyses inversion of a DNA segment permitting expression of a type IV pilin. However, the organization of IS110 family members and the inversion systems are different. In the IS, the recombinase is located within the element whereas in the inversion systems it is located outside the invertible segment [7].
Although it has proved difficult to determine the activity of these transposases in detail in vitro, transposition of ISs with DEDD Tpases may be unusual and involve Holliday Junction intermediates which must be resolved using a RuvC-like mechanism. This type of recombination would be consistent with the close relationship between DEDD transposases and the Piv/MooV invertases which presumably resolve Holliday Junction structures during inversion[116].
Chemistry of Serine-transposases
Other TE encode transposases which resemble serine site-specific recombinases. Their chemistry is presumably similar to that of their sequence-specific recombinase cousins. IS607 family (see ("IS607 family") members encode a Tpase closely resembling serine site-specific recombinases which use serine as a nucleophile for cleavage of the DNA strand. At present little is known about transposition of this IS family although it is thought that these elements generate circular intermediates (NDF Grindley pers. comm. cited in [117]). Presumably the enzyme catalyses similar cleavages and strand transfers as its site-specific serine recombinase cousins using a transitory 5’ phosphoserine covalent intermediate. Based on transposase structures from structural genomics studies and detailed knowledge of the general serine recombinase mechanism Boocock and Rice, [118] have proposed a model for the transposition mechanism. This includes a synaptic transposase tetramer (as for classical serine recombinases). The model explains the lack of target specificity exhibited in IS607 transposition[119], behavior which is unusual for this type of recombinase.
Bibliography
- ↑ Mizuuchi K . Polynucleotidyl transfer reactions in transpositional DNA recombination. - J Biol Chem: 1992 Oct 25, 267(30);21273-6 [PubMed:1383220]
- ↑ Mizuuchi K, Baker TA. Chemical mechanisms for mobilizing DNA. In: Craig NL, Craigie R, Gellert M, Lambowitz A, editors. Mobile DNA. Washington DC: ASM press; 2002. p. 12–23.
- ↑ Mizuuchi K . Polynucleotidyl transfer reactions in site-specific DNA recombination. - Genes Cells: 1997 Jan, 2(1);1-12 [PubMed:9112436] [DOI]
- ↑ 4.0 4.1 Hickman AB, Dyda F . Mechanisms of DNA Transposition. - Microbiol Spectr: 2015 Apr, 3(2);MDNA3-0034-2014 [PubMed:26104718] [DOI]
- ↑ Chandler M, de la Cruz F, Dyda F, Hickman AB, Moncalian G, Ton-Hoang B . Breaking and joining single-stranded DNA: the HUH endonuclease superfamily. - Nat Rev Microbiol: 2013 Aug, 11(8);525-38 [PubMed:23832240] [DOI]
- ↑ He S, Corneloup A, Guynet C, Lavatine L, Caumont-Sarcos A, Siguier P, Marty B, Dyda F, Chandler M, Ton Hoang B . The IS200/IS605 Family and "Peel and Paste" Single-strand Transposition Mechanism. - Microbiol Spectr: 2015 Aug, 3(4); [PubMed:26350330] [DOI]
- ↑ 7.0 7.1 Buchner JM, Robertson AE, Poynter DJ, Denniston SS, Karls AC . Piv site-specific invertase requires a DEDD motif analogous to the catalytic center of the RuvC Holliday junction resolvases. - J Bacteriol: 2005 May, 187(10);3431-7 [PubMed:15866929] [DOI]
- ↑ Ariyoshi M, Vassylyev DG, Iwasaki H, Nakamura H, Shinagawa H, Morikawa K . Atomic structure of the RuvC resolvase: a holliday junction-specific endonuclease from E. coli. - Cell: 1994 Sep 23, 78(6);1063-72 [PubMed:7923356] [DOI]
- ↑ Krupovic M, Makarova KS, Forterre P, Prangishvili D, Koonin EV . Casposons: a new superfamily of self-synthesizing DNA transposons at the origin of prokaryotic CRISPR-Cas immunity. - BMC Biol: 2014 May 19, 12;36 [PubMed:24884953] [DOI]
- ↑ Duval-Valentin G, Chandler M . Cotranslational control of DNA transposition: a window of opportunity. - Mol Cell: 2011 Dec 23, 44(6);989-96 [PubMed:22195971] [DOI]
- ↑ Mhammedi-Alaoui A, Pato M, Gama MJ, Toussaint A . A new component of bacteriophage Mu replicative transposition machinery: the Escherichia coli ClpX protein. - Mol Microbiol: 1994 Mar, 11(6);1109-16 [PubMed:8022280] [DOI]
- ↑ Kruklitis R, Welty DJ, Nakai H . ClpX protein of Escherichia coli activates bacteriophage Mu transposase in the strand transfer complex for initiation of Mu DNA synthesis. - EMBO J: 1996 Feb 15, 15(4);935-44 [PubMed:8631314]
- ↑ Levchenko I, Luo L, Baker TA . Disassembly of the Mu transposase tetramer by the ClpX chaperone. - Genes Dev: 1995 Oct 1, 9(19);2399-408 [PubMed:7557391] [DOI]
- ↑ Derbyshire KM, Kramer M, Grindley ND . Role of instability in the cis action of the insertion sequence IS903 transposase. - Proc Natl Acad Sci U S A: 1990 Jun, 87(11);4048-52 [PubMed:2161528] [DOI]
- ↑ Derbyshire KM, Grindley ND . Cis preference of the IS903 transposase is mediated by a combination of transposase instability and inefficient translation. - Mol Microbiol: 1996 Sep, 21(6);1261-72 [PubMed:8898394] [DOI]
- ↑ 16.0 16.1 Rice P, Craigie R, Davies DR . Retroviral integrases and their cousins. - Curr Opin Struct Biol: 1996 Feb, 6(1);76-83 [PubMed:8696976] [DOI]
- ↑ 17.0 17.1 Rice P, Mizuuchi K . Structure of the bacteriophage Mu transposase core: a common structural motif for DNA transposition and retroviral integration. - Cell: 1995 Jul 28, 82(2);209-20 [PubMed:7628012] [DOI]
- ↑ 18.0 18.1 Montaño SP, Rice PA . Moving DNA around: DNA transposition and retroviral integration. - Curr Opin Struct Biol: 2011 Jun, 21(3);370-8 [PubMed:21439812] [DOI]
- ↑ 19.0 19.1 Montaño SP, Pigli YZ, Rice PA . The μ transpososome structure sheds light on DDE recombinase evolution. - Nature: 2012 Nov 15, 491(7424);413-7 [PubMed:23135398] [DOI]
- ↑ Dyda F, Hickman AB, Jenkins TM, Engelman A, Craigie R, Davies DR . Crystal structure of the catalytic domain of HIV-1 integrase: similarity to other polynucleotidyl transferases. - Science: 1994 Dec 23, 266(5193);1981-6 [PubMed:7801124] [DOI]
- ↑ 21.0 21.1 Hickman AB, Chandler M, Dyda F . Integrating prokaryotes and eukaryotes: DNA transposases in light of structure. - Crit Rev Biochem Mol Biol: 2010 Feb, 45(1);50-69 [PubMed:20067338] [DOI]
- ↑ 22.0 22.1 Dyda F, Chandler M, Hickman AB . The emerging diversity of transpososome architectures. - Q Rev Biophys: 2012 Nov, 45(4);493-521 [PubMed:23217365] [DOI]
- ↑ Chow SA, Vincent KA, Ellison V, Brown PO . Reversal of integration and DNA splicing mediated by integrase of human immunodeficiency virus. - Science: 1992 Feb 7, 255(5045);723-6 [PubMed:1738845] [DOI]
- ↑ Polard P, Ton-Hoang B, Haren L, Bétermier M, Walczak R, Chandler M . IS911-mediated transpositional recombination in vitro. - J Mol Biol: 1996 Nov 22, 264(1);68-81 [PubMed:8950268] [DOI]
- ↑ Vos JC, van Luenen HG, Plasterk RH . Characterization of the Caenorhabditis elegans Tc1 transposase in vivo and in vitro. - Genes Dev: 1993 Jul, 7(7A);1244-53 [PubMed:8391505] [DOI]
- ↑ Engelman A, Mizuuchi K, Craigie R . HIV-1 DNA integration: mechanism of viral DNA cleavage and DNA strand transfer. - Cell: 1991 Dec 20, 67(6);1211-21 [PubMed:1760846] [DOI]
- ↑ Gerton JL, Herschlag D, Brown PO . Stereospecificity of reactions catalyzed by HIV-1 integrase. - J Biol Chem: 1999 Nov 19, 274(47);33480-7 [PubMed:10559232] [DOI]
- ↑ Mizuuchi K, Adzuma K . Inversion of the phosphate chirality at the target site of Mu DNA strand transfer: evidence for a one-step transesterification mechanism. - Cell: 1991 Jul 12, 66(1);129-40 [PubMed:1649006] [DOI]
- ↑ Kennedy AK, Haniford DB, Mizuuchi K . Single active site catalysis of the successive phosphoryl transfer steps by DNA transposases: insights from phosphorothioate stereoselectivity. - Cell: 2000 Apr 28, 101(3);295-305 [PubMed:10847684] [DOI]
- ↑ van Gent DC, Mizuuchi K, Gellert M . Similarities between initiation of V(D)J recombination and retroviral integration. - Science: 1996 Mar 15, 271(5255);1592-4 [PubMed:8599117] [DOI]
- ↑ Fayet O, Ramond P, Polard P, Prère MF, Chandler M . Functional similarities between retroviruses and the IS3 family of bacterial insertion sequences? - Mol Microbiol: 1990 Oct, 4(10);1771-7 [PubMed:1963920] [DOI]
- ↑ Kulkosky J, Jones KS, Katz RA, Mack JP, Skalka AM . Residues critical for retroviral integrative recombination in a region that is highly conserved among retroviral/retrotransposon integrases and bacterial insertion sequence transposases. - Mol Cell Biol: 1992 May, 12(5);2331-8 [PubMed:1314954] [DOI]
- ↑ Katzman M, Mack JP, Skalka AM, Leis J . A covalent complex between retroviral integrase and nicked substrate DNA. - Proc Natl Acad Sci U S A: 1991 Jun 1, 88(11);4695-9 [PubMed:1647013] [DOI]
- ↑ Yuan YW, Wessler SR . The catalytic domain of all eukaryotic cut-and-paste transposase superfamilies. - Proc Natl Acad Sci U S A: 2011 May 10, 108(19);7884-9 [PubMed:21518873] [DOI]
- ↑ 35.0 35.1 35.2 Rezsöhazy R, Hallet B, Delcour J, Mahillon J . The IS4 family of insertion sequences: evidence for a conserved transposase motif. - Mol Microbiol: 1993 Sep, 9(6);1283-95 [PubMed:7934941] [DOI]
- ↑ Polard P, Chandler M . Bacterial transposases and retroviral integrases. - Mol Microbiol: 1995 Jan, 15(1);13-23 [PubMed:7752887] [DOI]
- ↑ 37.0 37.1 37.2 Jenkins TM, Esposito D, Engelman A, Craigie R . Critical contacts between HIV-1 integrase and viral DNA identified by structure-based analysis and photo-crosslinking. - EMBO J: 1997 Nov 17, 16(22);6849-59 [PubMed:9362498] [DOI]
- ↑ Doak TG, Doerder FP, Jahn CL, Herrick G . A proposed superfamily of transposase genes: transposon-like elements in ciliated protozoa and a common "D35E" motif. - Proc Natl Acad Sci U S A: 1994 Feb 1, 91(3);942-6 [PubMed:8302872] [DOI]
- ↑ 39.0 39.1 Esposito D, Craigie R . Sequence specificity of viral end DNA binding by HIV-1 integrase reveals critical regions for protein-DNA interaction. - EMBO J: 1998 Oct 1, 17(19);5832-43 [PubMed:9755183] [DOI]
- ↑ Goldgur Y, Dyda F, Hickman AB, Jenkins TM, Craigie R, Davies DR . Three new structures of the core domain of HIV-1 integrase: an active site that binds magnesium. - Proc Natl Acad Sci U S A: 1998 Aug 4, 95(16);9150-4 [PubMed:9689049] [DOI]
- ↑ Haren L, Ton-Hoang B, Chandler M . Integrating DNA: transposases and retroviral integrases. - Annu Rev Microbiol: 1999, 53;245-81 [PubMed:10547692] [DOI]
- ↑ 42.0 42.1 Gerton JL, Ohgi S, Olsen M, DeRisi J, Brown PO . Effects of mutations in residues near the active site of human immunodeficiency virus type 1 integrase on specific enzyme-substrate interactions. - J Virol: 1998 Jun, 72(6);5046-55 [PubMed:9573274] [DOI]
- ↑ 43.0 43.1 Engelman A, Cherepanov P . Retroviral Integrase Structure and DNA Recombination Mechanism. - Microbiol Spectr: 2014 Dec, 2(6); [PubMed:26104441] [DOI]
- ↑ Bolland S, Kleckner N . The three chemical steps of Tn10/IS10 transposition involve repeated utilization of a single active site. - Cell: 1996 Jan 26, 84(2);223-33 [PubMed:8565068] [DOI]
- ↑ Reznikoff WS . Transposon Tn5. - Annu Rev Genet: 2008, 42;269-86 [PubMed:18680433] [DOI]
- ↑ Tavakoli NP, DeVost J, Derbyshire KM . Defining functional regions of the IS903 transposase. - J Mol Biol: 1997 Dec 12, 274(4);491-504 [PubMed:9417930] [DOI]
- ↑ 47.0 47.1 Berg DE . Structural requirement for IS50-mediated gene transposition. - Proc Natl Acad Sci U S A: 1983 Feb, 80(3);792-6 [PubMed:6298776] [DOI]
- ↑ Lichens-Park A, Syvanen M . Cointegrate formation by IS50 requires multiple donor molecules. - Mol Gen Genet: 1988 Feb, 211(2);244-51 [PubMed:2832702] [DOI]
- ↑ Kiss J, Olasz F . Formation and transposition of the covalently closed IS30 circle: the relation between tandem dimers and monomeric circles. - Mol Microbiol: 1999 Oct, 34(1);37-52 [PubMed:10540284] [DOI]
- ↑ Olasz F, Farkas T, Kiss J, Arini A, Arber W . Terminal inverted repeats of insertion sequence IS30 serve as targets for transposition. - J Bacteriol: 1997 Dec, 179(23);7551-8 [PubMed:9393723] [DOI]
- ↑ Reimmann C, Haas D . Mode of replicon fusion mediated by the duplicated insertion sequence IS21 in Escherichia coli. - Genetics: 1987 Apr, 115(4);619-25 [PubMed:3034717] [DOI]
- ↑ Reimmann C, Rella M, Haas D . Integration of replication-defective R68.45-like plasmids into the Pseudomonas aeruginosa chromosome. - J Gen Microbiol: 1988 Jun, 134(6);1515-23 [PubMed:3146616] [DOI]
- ↑ Spielmann-Ryser J, Moser M, Kast P, Weber H . Factors determining the frequency of plasmid cointegrate formation mediated by insertion sequence IS3 from Escherichia coli. - Mol Gen Genet: 1991 May, 226(3);441-8 [PubMed:1645443] [DOI]
- ↑ Turlan C, Ton-Hoang B, Chandler M . The role of tandem IS dimers in IS911 transposition. - Mol Microbiol: 2000 Mar, 35(6);1312-25 [PubMed:10760133] [DOI]
- ↑ Davies DR, Mahnke Braam L, Reznikoff WS, Rayment I . The three-dimensional structure of a Tn5 transposase-related protein determined to 2.9-A resolution. - J Biol Chem: 1999 Apr 23, 274(17);11904-13 [PubMed:10207011] [DOI]
- ↑ Davies DR, Goryshin IY, Reznikoff WS, Rayment I . Three-dimensional structure of the Tn5 synaptic complex transposition intermediate. - Science: 2000 Jul 7, 289(5476);77-85 [PubMed:10884228] [DOI]
- ↑ Valkov E, Gupta SS, Hare S, Helander A, Roversi P, McClure M, Cherepanov P . Functional and structural characterization of the integrase from the prototype foamy virus. - Nucleic Acids Res: 2009 Jan, 37(1);243-55 [PubMed:19036793] [DOI]
- ↑ Hare S, Di Nunzio F, Labeja A, Wang J, Engelman A, Cherepanov P . Structural basis for functional tetramerization of lentiviral integrase. - PLoS Pathog: 2009 Jul, 5(7);e1000515 [PubMed:19609359] [DOI]
- ↑ Hare S, Shun MC, Gupta SS, Valkov E, Engelman A, Cherepanov P . A novel co-crystal structure affords the design of gain-of-function lentiviral integrase mutants in the presence of modified PSIP1/LEDGF/p75. - PLoS Pathog: 2009 Jan, 5(1);e1000259 [PubMed:19132083] [DOI]
- ↑ Li X, Krishnan L, Cherepanov P, Engelman A . Structural biology of retroviral DNA integration. - Virology: 2011 Mar 15, 411(2);194-205 [PubMed:21216426] [DOI]
- ↑ Cherepanov P, Maertens GN, Hare S . Structural insights into the retroviral DNA integration apparatus. - Curr Opin Struct Biol: 2011 Apr, 21(2);249-56 [PubMed:21277766] [DOI]
- ↑ Cherepanov P . Integrase illuminated. - EMBO Rep: 2010 May, 11(5);328 [PubMed:20428106] [DOI]
- ↑ Maskell DP, Renault L, Serrao E, Lesbats P, Matadeen R, Hare S, Lindemann D, Engelman AN, Costa A, Cherepanov P . Structural basis for retroviral integration into nucleosomes. - Nature: 2015 Jul 16, 523(7560);366-9 [PubMed:26061770] [DOI]
- ↑ Richardson JM, Zhang L, Marcos S, Finnegan DJ, Harding MM, Taylor P, Walkinshaw MD . Expression, purification and preliminary crystallographic studies of a single-point mutant of Mos1 mariner transposase. - Acta Crystallogr D Biol Crystallogr: 2004 May, 60(Pt 5);962-4 [PubMed:15103153] [DOI]
- ↑ Richardson JM, Finnegan DJ, Walkinshaw MD . Crystallization of a Mos1 transposase-inverted-repeat DNA complex: biochemical and preliminary crystallographic analyses. - Acta Crystallogr Sect F Struct Biol Cryst Commun: 2007 May 1, 63(Pt 5);434-7 [PubMed:17565190] [DOI]
- ↑ Richardson JM, Colloms SD, Finnegan DJ, Walkinshaw MD . Molecular architecture of the Mos1 paired-end complex: the structural basis of DNA transposition in a eukaryote. - Cell: 2009 Sep 18, 138(6);1096-108 [PubMed:19766564] [DOI]
- ↑ Cuypers MG, Trubitsyna M, Callow P, Forsyth VT, Richardson JM . Solution conformations of early intermediates in Mos1 transposition. - Nucleic Acids Res: 2013 Feb 1, 41(3);2020-33 [PubMed:23262225] [DOI]
- ↑ Perez ZN, Musingarimi P, Craig NL, Dyda F, Hickman AB . Purification, crystallization and preliminary crystallographic analysis of the Hermes transposase. - Acta Crystallogr Sect F Struct Biol Cryst Commun: 2005 Jun 1, 61(Pt 6);587-90 [PubMed:16511103] [DOI]
- ↑ Hickman AB, Perez ZN, Zhou L, Musingarimi P, Ghirlando R, Hinshaw JE, Craig NL, Dyda F . Molecular architecture of a eukaryotic DNA transposase. - Nat Struct Mol Biol: 2005 Aug, 12(8);715-21 [PubMed:16041385] [DOI]
- ↑ Hickman AB, Ewis HE, Li X, Knapp JA, Laver T, Doss AL, Tolun G, Steven AC, Grishaev A, Bax A, Atkinson PW, Craig NL, Dyda F . Structural basis of hAT transposon end recognition by Hermes, an octameric DNA transposase from Musca domestica. - Cell: 2014 Jul 17, 158(2);353-367 [PubMed:25036632] [DOI]
- ↑ Naumann TA, Reznikoff WS . Trans catalysis in Tn5 transposition. - Proc Natl Acad Sci U S A: 2000 Aug 1, 97(16);8944-9 [PubMed:10908658] [DOI]
- ↑ Savilahti H, Mizuuchi K . Mu transpositional recombination: donor DNA cleavage and strand transfer in trans by the Mu transposase. - Cell: 1996 Apr 19, 85(2);271-80 [PubMed:8612279] [DOI]
- ↑ Aldaz H, Schuster E, Baker TA . The interwoven architecture of the Mu transposase couples DNA synapsis to catalysis. - Cell: 1996 Apr 19, 85(2);257-69 [PubMed:8612278] [DOI]
- ↑ Dornan J, Grey H, Richardson JM . Structural role of the flanking DNA in mariner transposon excision. - Nucleic Acids Res: 2015 Feb 27, 43(4);2424-32 [PubMed:25662605] [DOI]
- ↑ Maertens GN, Hare S, Cherepanov P . The mechanism of retroviral integration from X-ray structures of its key intermediates. - Nature: 2010 Nov 11, 468(7321);326-9 [PubMed:21068843] [DOI]
- ↑ Hare S, Gupta SS, Valkov E, Engelman A, Cherepanov P . Retroviral intasome assembly and inhibition of DNA strand transfer. - Nature: 2010 Mar 11, 464(7286);232-6 [PubMed:20118915] [DOI]
- ↑ Ilyina TV, Koonin EV . Conserved sequence motifs in the initiator proteins for rolling circle DNA replication encoded by diverse replicons from eubacteria, eucaryotes and archaebacteria. - Nucleic Acids Res: 1992 Jul 11, 20(13);3279-85 [PubMed:1630899] [DOI]
- ↑ 78.0 78.1 Koonin EV, Ilyina TV . Computer-assisted dissection of rolling circle DNA replication. - Biosystems: 1993, 30(1-3);241-68 [PubMed:8374079] [DOI]
- ↑ 79.0 79.1 79.2 79.3 Kapitonov VV, Jurka J . Rolling-circle transposons in eukaryotes. - Proc Natl Acad Sci U S A: 2001 Jul 17, 98(15);8714-9 [PubMed:11447285] [DOI]
- ↑ 80.0 80.1 80.2 80.3 Ton-Hoang B, Guynet C, Ronning DR, Cointin-Marty B, Dyda F, Chandler M . Transposition of ISHp608, member of an unusual family of bacterial insertion sequences. - EMBO J: 2005 Sep 21, 24(18);3325-38 [PubMed:16163392] [DOI]
- ↑ 81.0 81.1 81.2 81.3 Ronning DR, Guynet C, Ton-Hoang B, Perez ZN, Ghirlando R, Chandler M, Dyda F . Active site sharing and subterminal hairpin recognition in a new class of DNA transposases. - Mol Cell: 2005 Oct 7, 20(1);143-54 [PubMed:16209952] [DOI]
- ↑ 82.0 82.1 82.2 82.3 Toleman MA, Bennett PM, Walsh TR . ISCR elements: novel gene-capturing systems of the 21st century? - Microbiol Mol Biol Rev: 2006 Jun, 70(2);296-316 [PubMed:16760305] [DOI]
- ↑ Garcillán-Barcia MP, Francia MV, de la Cruz F . The diversity of conjugative relaxases and its application in plasmid classification. - FEMS Microbiol Rev: 2009 May, 33(3);657-87 [PubMed:19396961] [DOI]
- ↑ Gruss A, Ehrlich SD . The family of highly interrelated single-stranded deoxyribonucleic acid plasmids. - Microbiol Rev: 1989 Jun, 53(2);231-41 [PubMed:2666843] [DOI]
- ↑ Rosario K, Duffy S, Breitbart M . A field guide to eukaryotic circular single-stranded DNA viruses: insights gained from metagenomics. - Arch Virol: 2012 Oct, 157(10);1851-71 [PubMed:22760663] [DOI]
- ↑ de la Cruz F, Frost LS, Meyer RJ, Zechner EL . Conjugative DNA metabolism in Gram-negative bacteria. - FEMS Microbiol Rev: 2010 Jan, 34(1);18-40 [PubMed:19919603] [DOI]
- ↑ Guglielmini J, Quintais L, Garcillán-Barcia MP, de la Cruz F, Rocha EP . The repertoire of ICE in prokaryotes underscores the unity, diversity, and ubiquity of conjugation. - PLoS Genet: 2011 Aug, 7(8);e1002222 [PubMed:21876676] [DOI]
- ↑ Odegrip R, Haggård-Ljungquist E . The two active-site tyrosine residues of the a protein play non-equivalent roles during initiation of rolling circle replication of bacteriophage p2. - J Mol Biol: 2001 Apr 27, 308(2);147-63 [PubMed:11327759] [DOI]
- ↑ Grindley ND, Whiteson KL, Rice PA . Mechanisms of site-specific recombination. - Annu Rev Biochem: 2006, 75;567-605 [PubMed:16756503] [DOI]
- ↑ Hickman AB, James JA, Barabas O, Pasternak C, Ton-Hoang B, Chandler M, Sommer S, Dyda F . DNA recognition and the precleavage state during single-stranded DNA transposition in D. radiodurans. - EMBO J: 2010 Nov 17, 29(22);3840-52 [PubMed:20890269] [DOI]
- ↑ 91.0 91.1 91.2 Boer R, Russi S, Guasch A, Lucas M, Blanco AG, Pérez-Luque R, Coll M, de la Cruz F . Unveiling the molecular mechanism of a conjugative relaxase: The structure of TrwC complexed with a 27-mer DNA comprising the recognition hairpin and the cleavage site. - J Mol Biol: 2006 May 5, 358(3);857-69 [PubMed:16540117] [DOI]
- ↑ 92.0 92.1 Boer DR, Ruíz-Masó JA, López-Blanco JR, Blanco AG, Vives-Llàcer M, Chacón P, Usón I, Gomis-Rüth FX, Espinosa M, Llorca O, del Solar G, Coll M . Plasmid replication initiator RepB forms a hexamer reminiscent of ring helicases and has mobile nuclease domains. - EMBO J: 2009 Jun 3, 28(11);1666-78 [PubMed:19440202] [DOI]
- ↑ 93.0 93.1 Datta S, Larkin C, Schildbach JF . Structural insights into single-stranded DNA binding and cleavage by F factor TraI. - Structure: 2003 Nov, 11(11);1369-79 [PubMed:14604527] [DOI]
- ↑ Larkin C, Datta S, Harley MJ, Anderson BJ, Ebie A, Hargreaves V, Schildbach JF . Inter- and intramolecular determinants of the specificity of single-stranded DNA binding and cleavage by the F factor relaxase. - Structure: 2005 Oct, 13(10);1533-44 [PubMed:16216584] [DOI]
- ↑ 95.0 95.1 95.2 Edwards JS, Betts L, Frazier ML, Pollet RM, Kwong SM, Walton WG, Ballentine WK 3rd, Huang JJ, Habibi S, Del Campo M, Meier JL, Dervan PB, Firth N, Redinbo MR . Molecular basis of antibiotic multiresistance transfer in Staphylococcus aureus. - Proc Natl Acad Sci U S A: 2013 Feb 19, 110(8);2804-9 [PubMed:23359708] [DOI]
- ↑ 96.0 96.1 96.2 96.3 Messing SA, Ton-Hoang B, Hickman AB, McCubbin AJ, Peaslee GF, Ghirlando R, Chandler M, Dyda F . The processing of repetitive extragenic palindromes: the structure of a repetitive extragenic palindrome bound to its associated nuclease. - Nucleic Acids Res: 2012 Oct, 40(19);9964-79 [PubMed:22885300] [DOI]
- ↑ 97.0 97.1 97.2 Hickman AB, Ronning DR, Perez ZN, Kotin RM, Dyda F . The nuclease domain of adeno-associated virus rep coordinates replication initiation using two distinct DNA recognition interfaces. - Mol Cell: 2004 Feb 13, 13(3);403-14 [PubMed:14967147] [DOI]
- ↑ 98.0 98.1 98.2 98.3 Guasch A, Lucas M, Moncalián G, Cabezas M, Pérez-Luque R, Gomis-Rüth FX, de la Cruz F, Coll M . Recognition and processing of the origin of transfer DNA by conjugative relaxase TrwC. - Nat Struct Biol: 2003 Dec, 10(12);1002-10 [PubMed:14625590] [DOI]
- ↑ 99.0 99.1 Larkin C, Datta S, Nezami A, Dohm JA, Schildbach JF . Crystallization and preliminary X-ray characterization of the relaxase domain of F factor TraI. - Acta Crystallogr D Biol Crystallogr: 2003 Aug, 59(Pt 8);1514-6 [PubMed:12876370] [DOI]
- ↑ Dyda F, Hickman AB . A mob of reps. - Structure: 2003 Nov, 11(11);1310-1 [PubMed:14604517] [DOI]
- ↑ Clérot D, Bernardi F . DNA helicase activity is associated with the replication initiator protein rep of tomato yellow leaf curl geminivirus. - J Virol: 2006 Nov, 80(22);11322-30 [PubMed:16943286] [DOI]
- ↑ Brister JR, Muzyczka N . Rep-mediated nicking of the adeno-associated virus origin requires two biochemical activities, DNA helicase activity and transesterification. - J Virol: 1999 Nov, 73(11);9325-36 [PubMed:10516041] [DOI]
- ↑ Im DS, Muzyczka N . The AAV origin binding protein Rep68 is an ATP-dependent site-specific endonuclease with DNA helicase activity. - Cell: 1990 May 4, 61(3);447-57 [PubMed:2159383] [DOI]
- ↑ Brister JR, Muzyczka N . Mechanism of Rep-mediated adeno-associated virus origin nicking. - J Virol: 2000 Sep, 74(17);7762-71 [PubMed:10933682] [DOI]
- ↑ Ton-Hoang B, Siguier P, Quentin Y, Onillon S, Marty B, Fichant G, Chandler M . Structuring the bacterial genome: Y1-transposases associated with REP-BIME sequences. - Nucleic Acids Res: 2012 Apr, 40(8);3596-609 [PubMed:22199259] [DOI]
- ↑ Orozco BM, Hanley-Bowdoin L . A DNA structure is required for geminivirus replication origin function. - J Virol: 1996 Jan, 70(1);148-58 [PubMed:8523519] [DOI]
- ↑ del Solar G, Giraldo R, Ruiz-Echevarría MJ, Espinosa M, Díaz-Orejas R . Replication and control of circular bacterial plasmids. - Microbiol Mol Biol Rev: 1998 Jun, 62(2);434-64 [PubMed:9618448] [DOI]
- ↑ 108.0 108.1 Bikard D, Loot C, Baharoglu Z, Mazel D . Folded DNA in action: hairpin formation and biological functions in prokaryotes. - Microbiol Mol Biol Rev: 2010 Dec, 74(4);570-88 [PubMed:21119018] [DOI]
- ↑ Ruiz-Masó JA, Lurz R, Espinosa M, del Solar G . Interactions between the RepB initiator protein of plasmid pMV158 and two distant DNA regions within the origin of replication. - Nucleic Acids Res: 2007, 35(4);1230-44 [PubMed:17267412] [DOI]
- ↑ Mendiola MV, Bernales I, de la Cruz F . Differential roles of the transposon termini in IS91 transposition. - Proc Natl Acad Sci U S A: 1994 Mar 1, 91(5);1922-6 [PubMed:8127907] [DOI]
- ↑ Tobiason DM, Buchner JM, Thiel WH, Gernert KM, Karls AC . Conserved amino acid motifs from the novel Piv/MooV family of transposases and site-specific recombinases are required for catalysis of DNA inversion by Piv. - Mol Microbiol: 2001 Feb, 39(3);641-51 [PubMed:11169105] [DOI]
- ↑ Fulks KA, Marrs CF, Stevens SP, Green MR . Sequence analysis of the inversion region containing the pilin genes of Moraxella bovis. - J Bacteriol: 1990 Jan, 172(1);310-6 [PubMed:2403542] [DOI]
- ↑ Rozsa FW, Meyer TF, Fussenegger M . Inversion of Moraxella lacunata type 4 pilin gene sequences by a Neisseria gonorrhoeae site-specific recombinase. - J Bacteriol: 1997 Apr, 179(7);2382-8 [PubMed:9079926] [DOI]
- ↑ Skaar EP, Lecuyer B, Lenich AG, Lazio MP, Perkins-Balding D, Seifert HS, Karls AC . Analysis of the Piv recombinase-related gene family of Neisseria gonorrhoeae. - J Bacteriol: 2005 Feb, 187(4);1276-86 [PubMed:15687191] [DOI]
- ↑ Choi S, Ohta S, Ohtsubo E . A novel IS element, IS621, of the IS110/IS492 family transposes to a specific site in repetitive extragenic palindromic sequences in Escherichia coli. - J Bacteriol: 2003 Aug, 185(16);4891-900 [PubMed:12897009] [DOI]
- ↑ Tobiason DM, Lenich AG, Glasgow AC . Multiple DNA binding activities of the novel site-specific recombinase, Piv, from Moraxella lacunata. - J Biol Chem: 1999 Apr 2, 274(14);9698-706 [PubMed:10092658] [DOI]
- ↑ Filée J, Siguier P, Chandler M . Insertion sequence diversity in archaea. - Microbiol Mol Biol Rev: 2007 Mar, 71(1);121-57 [PubMed:17347521] [DOI]
- ↑ Boocock MR, Rice PA . A proposed mechanism for IS607-family serine transposases. - Mob DNA: 2013 Nov 6, 4(1);24 [PubMed:24195768] [DOI]
- ↑ Kersulyte D, Mukhopadhyay AK, Shirai M, Nakazawa T, Berg DE . Functional organization and insertion specificity of IS607, a chimeric element of Helicobacter pylori. - J Bacteriol: 2000 Oct, 182(19);5300-8 [PubMed:10986230] [DOI]