General Information/What Is an IS?
Contents
Classical IS
The original definition of an IS (Fig.3.1) was: a short, generally phenotypically cryptic, DNA segment encoding only the enzymes necessary for its transposition and capable of repeated insertion into many different sites within a genome using mechanisms independent of large regions of DNA homology between the IS and target [1][2][3]. Classical IS are between 0.7 and 2.5 kb in length, genetically compact with one or two open reading frames (orfs) which occupy the entire length of the IS and terminate in flanking imperfect terminal repeat sequences (IR) (Table 1). The orfs include the Tpase that catalyzes the DNA cleavages and strand transfers leading to IS movement and, in some cases, regulatory proteins. Their highly compact nature is illustrated by the fact that some IS have developed “recoding” strategies such as Programmed Ribosomal Frameshifting (involving ribosome slippage) and Programmed Transcriptional Realignment (involving RNA polymerase slippage) [4][5][6][7][8][9][10][11].
These permit assembly of different functional protein domains, effectively encoding two proteins of different functions in one DNA segment. IS also often generates a short flanking directly repeated duplication (DR) of the target DNA on insertion. These characteristics are not limited to prokaryotic IS but are also shared with most eukaryotic DNA transposons. Classical IS generally transpose using a double-strand DNA intermediate. However, for prokaryotic IS, this strict definition has been broadened over the years with the discovery of an increasing number of non-canonical derivatives and variants, some of which are described in the following sections. Moreover, as we learn more about diversity from sequenced genomes, classification is becoming more problematic because the large degree of MGE diversity is obscuring the borders between certain types of TE (see "Fuzzy Borders") [5]. Despite their abundance and diversity, the number of different chemical mechanisms used in TE movement is surprisingly limited, and many quite divergent TE share a similar mechanism.
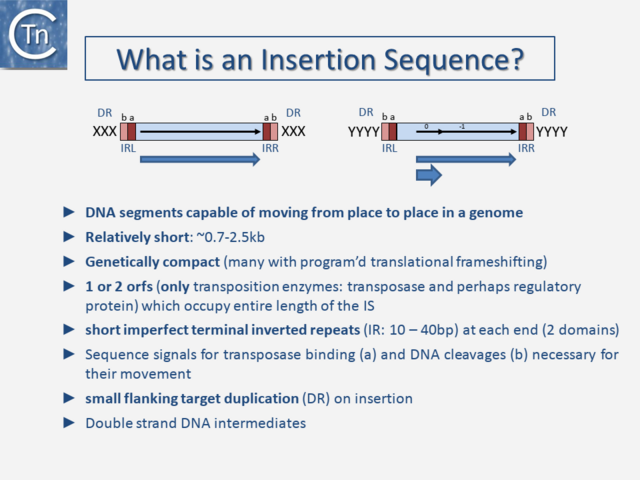
Characteristics of insertion sequence families
| Table 1. Abbreviations: DR, duplication repeat; IS, insertion sequence; ORF, open reading frame. | |||||||||
| Families | Sub-Groups | Typical size-range (bp) | DR (bp) | Ends | IRs | No ORFs | Frameshift | Catalytic residues | Mechanism |
|---|---|---|---|---|---|---|---|---|---|
| IS1 | — | 740–1180 | 8–9 | GGnnnTG | Y | 2 | ORFAB | DDE | copy-and-paste and cointegrate |
| single ORF | 800–1200 | 0–9 | N | 1 | — | ||||
| ISMhu11 | 900–4600 | 0–10 | Y | 2 | ORFAB | ||||
| IS1595 | ISPna2 | 1000–1150 | 8 | GGCnnTG | Y | 1 | — | DDNK | copy-and-paste (?) |
| ISPna2+pass | 1500–2600 | 8 | — | 1+pass | — | ||||
| ISH4 | 1000 | 8 | CGCTCTT | 1 | DDNK | ||||
| IS1016 | 700–745 | 7–9 | GGGgctg | DDEK | |||||
| IS1595 | 900–1100 | 8 | CcTGATT | DDNK+ER4R7 | |||||
| ISSod11 | 1000–1100 | 8 | nnnGcnTATC | DDHK+ER4R7 | |||||
| ISNwi1 | 1080–1200 | 8 | ggnnatTAT | DDEK+ER4 | |||||
| ISNwi1+pass | 1750–4750 | 8 | — | 1+pass | — | ||||
| ISNha5 | 3450–7900 | 8 | CGGnnTT | 1 | DDER/K | ||||
| IS3 | IS150 | 1200–1600 | 3–4 | TG | Y | 2 | ORFAB | DDE | copy-and-paste |
| IS407 | 1100–1400 | 4 | TG | ||||||
| IS51 | 1000–1400 | 3–4 | TG | ||||||
| IS3 | 1150–1750 | 3–4 | TGa/g | ||||||
| IS2 | 1300–1400 | 5 | TG | ||||||
| IS481 | — | 950–1300 | 4–15 | TGT | Y | 1 | — | DDE | copy-and-paste (?) |
| IS1202 | — | 1400–1700 | 5 | TGT | Y | 1 | — | DDE | — |
| IS4 | IS10 | 1200–1350 | 9 | CT | Y | 1 | DDE | hairpin intermediate | cut-and-paste |
| IS50 | 1350–1550 | 8–9 | C | hairpin intermediate | |||||
| ISPepr1 | 1500–1600 | 7–8 | -T-AA | ? | |||||
| IS4 | 1400–1600 | 10–13 | -AAT | ? | |||||
| IS4Sa | 1150–1750 | 8–10 | CA | ? | |||||
| ISH8 | 1400–1800 | 10 | ? | ||||||
| IS231 | 1450–5400 | 10–12 | CAT | 1 or + * | *passenger genes | ||||
| IS701 | — | 1400–1550 | 4 | — | Y | 1 | — | DDE | — |
| ISAba11 | — | — | |||||||
| ISH3 | — | 1225–1500 | 4–5 | C-GT | Y | 1 | — | DDE | — |
| IS1634 | — | 1500–2000 | 5–6 | C | Y | 1 | — | DDE | — |
| IS5 | IS903 | 950–1150 | 9 | GG | Y | 1 | — | DDE | — |
| ISL2 | 850–1200 | 2–3 | — | ||||||
| ISH1 | 900–1150 | 8 | -GC | ||||||
| IS5 | 1000–1500 | 4 | Ga/g | ||||||
| IS1031 | 850–1050 | 3 | GAa/g | ||||||
| IS427 | 800–1000 | 2–4 | Ga/g | 2 | ORFAB | ||||
| IS1182 | — | 1330–1950 | 0–60 | — | Y | 1 | — | DDE | — |
| IS6 | — | 700–900 | 8 | GG | Y | 1 | — | DDE | co-integrate |
| IS21 | — | 1750–2600 | 4–8 | TG | Y | 2 * | — | DDE | — |
| IS30 | — | 1000–1700 | 2–3 | — | Y | 1 | — | DDE | copy-and-paste |
| IS66 | — | 2000–3000 | 8–9 | GTAA | Y | 3* | — | DDE* | — |
| ISBst12 | 1350–1900 | 1 | DDE | ||||||
| IS256 | — | 1200–1500 | 8–9 | Ga/g | Y | 1 | — | DDE | copy-and-paste |
| IS1249 | 1300 | 0–10 | GG | ||||||
| ISC1250 | 1250 | 0–9 | GG | ||||||
| ISH6 | — | 1450 | 8 | GGT | Y | 1 | — | DDE | — |
| ISLre2 | — | 1500–2000 | 9 | — | Y | 1 | — | DDE | — |
| ISKra4 | ISAzba1 | 1400–2900 | 0 | — | Y | 1 or + * | — | DDE | — |
| ISMich2 | 1250–1400 | 8 | GGG | 1 or 2 | ORFAB | ||||
| ISKra4 | 1400–3700 | 9 | GGG | 1 or + * | — | ||||
| IS630 | — | 1000–1400 | 2* | — | Y | 1 or 2 | ORFAB | DDE | cut-and-paste |
| IS982 | — | 1000 | 3–9 | AC | Y | 1 | — | DDE | — |
| IS1380 | — | 1550–2000 | 4–5 | CC | Y | 1 | — | DDE | — |
| ISAs1 | — | 1200–1500 | 8–10 | CAGGG | Y | 1 | — | — | — |
| ISL3 | — | 1300–2300 | 8 | GG | Y | 1 | — | — | — |
| Tn3 | — | >3000 | 0 | GGGG | Y | >1 | — | DDE | co-integrate |
| ISAzo13 | — | 1250–2200 | 0–4 | Ga/g | Y | 1 | — | — | — |
| IS110 | — | 1200–1550 | 0 | — | N | 1 | — | DEDD | — |
| IS1111 | — | — | — | Y* | — | — | — | — | |
| IS91 | — | 1500–2000 | 0 | — | N | 1 | — | HUH/Y2 | rolling-circle |
| IS200/IS605 | IS200 | 600–750 | 0 | — | 0 | 1* | — | HUH/Y1 | peel-and-paste |
| IS605 | 1300–2000 | — | — | — | 2* | — | HUH/Y1** | ||
| IS607 | — | 1700–2500 | 0 | — | N | 2* | — | Serine** | — |
| ISNCY * | IS892 | 1600 | 0–8 | CTAG | Y | 2 | ORFAB | — | — |
| ISLbi1 | 1400–1500 | 5 | — | Y | 1 | ||||
| ISMae2 | 1400–2400 | 9 | CAG | Y | 1 | ||||
| ISPlu15 | 800–1000 | 0 | — | N | 1 | ||||
| ISA1214 | 1000–1200 | 8–12 | — | Y | 2 | ||||
| ISC1217 | 1200 | 6–8 | TAG | Y | 1 | ||||
| ISM1 | 1300–1600 | 8–9 | — | Y | 1 | ||||
| IS1202 | 1400–1700 | 5 | TGT | Y | 1 | — | DDEQ | — | |
| ISDol1 | 1600–1900 | 6–7 | — | Y | 1 | — | DDE | — | |
New types of IS
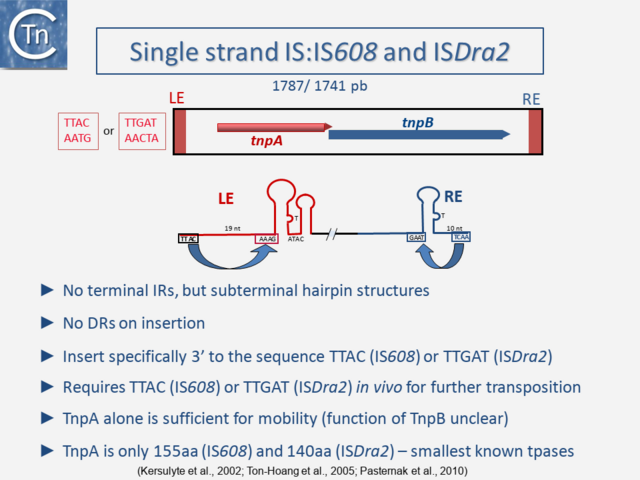
One example of this expanding diversity is the identification of another entire class of IS [12][13][14]. Members of this class use an entirely different mechanism of transposition involving single-strand circular DNA intermediates which appear to target stalled replication forks [15] (Fig.3.2). They possess small transposases (~150 aa) which are completely different to the classical IS in the type of chemistry they catalyze (Groups with HUH Enzymes). Another example are the casposons which are related to CRISPRs but whose transposition has yet to be fully characterized [16][17][18][19].
Bibliography
- ↑ Siguier P, Gourbeyre E, Varani A, Ton-Hoang B, Chandler M . Everyman's Guide to Bacterial Insertion Sequences. - Microbiol Spectr: 2015 Apr, 3(2);MDNA3-0030-2014 [PubMed:26104715] [DOI]
- ↑ Craig NL, Lambowitz AM, Craigie R, Gellert M, editors. Mobile DNA II. American Society of Microbiology; 2002.
- ↑ Craig, Chandler, Gellert, Lambowitz, Rice, Sandmeyer, editors. Mobile DNA III. American Society of Microbiology; 2015.
- ↑ Sharma V, Firth AE, Antonov I, Fayet O, Atkins JF, Borodovsky M, Baranov PV . A pilot study of bacterial genes with disrupted ORFs reveals a surprising profusion of protein sequence recoding mediated by ribosomal frameshifting and transcriptional realignment. - Mol Biol Evol: 2011 Nov, 28(11);3195-211 [PubMed:21673094] [DOI]
- ↑ 5.0 5.1 Siguier P, Gourbeyre E, Chandler M . Bacterial insertion sequences: their genomic impact and diversity. - FEMS Microbiol Rev: 2014 Sep, 38(5);865-91 [PubMed:24499397] [DOI]
- ↑ Chandler M, Fayet O, Rousseau P, Ton Hoang B, Duval-Valentin G . Copy-out-Paste-in Transposition of IS911: A Major Transposition Pathway. - Microbiol Spectr: 2015 Aug, 3(4); [PubMed:26350305] [DOI]
- ↑ Fayet O, Prère MF. Programmed Ribosomal −1 Frameshifting as a Tradition: The Bacterial Transposable Elements of the IS3 Family. In: Atkins JF, Gesteland RF, editors. Recoding: Expansion of Decoding Rules Enriches Gene Expression. New York and Heidelberg: Springer; 2010. p. 259–280.
- ↑ Prère MF, Canal I, Wills NM, Atkins JF, Fayet O . The interplay of mRNA stimulatory signals required for AUU-mediated initiation and programmed -1 ribosomal frameshifting in decoding of transposable element IS911. - J Bacteriol: 2011 Jun, 193(11);2735-44 [PubMed:21478364] [DOI]
- ↑ Baranov PV, Gurvich OL, Fayet O, Prère MF, Miller WA, Gesteland RF, Atkins JF, Giddings MC . RECODE: a database of frameshifting, bypassing and codon redefinition utilized for gene expression. - Nucleic Acids Res: 2001 Jan 1, 29(1);264-7 [PubMed:11125107] [DOI]
- ↑ Chandler M, Fayet O . Translational frameshifting in the control of transposition in bacteria. - Mol Microbiol: 1993 Feb, 7(4);497-503 [PubMed:8384687] [DOI]
- ↑ Atkins JF, Baranov PV, Fayet O, Herr AJ, Howard MT, Ivanov IP, Matsufuji S, Miller WA, Moore B, Prère MF, Wills NM, Zhou J, Gesteland RF . Overriding standard decoding: implications of recoding for ribosome function and enrichment of gene expression. - Cold Spring Harb Symp Quant Biol: 2001, 66;217-32 [PubMed:12762024] [DOI]
- ↑ Kersulyte D, Mukhopadhyay AK, Shirai M, Nakazawa T, Berg DE . Functional organization and insertion specificity of IS607, a chimeric element of Helicobacter pylori. - J Bacteriol: 2000 Oct, 182(19);5300-8 [PubMed:10986230] [DOI]
- ↑ Kersulyte D, Akopyants NS, Clifton SW, Roe BA, Berg DE . Novel sequence organization and insertion specificity of IS605 and IS606: chimaeric transposable elements of Helicobacter pylori. - Gene: 1998 Nov 26, 223(1-2);175-86 [PubMed:9858724] [DOI]
- ↑ Kersulyte D, Velapatiño B, Dailide G, Mukhopadhyay AK, Ito Y, Cahuayme L, Parkinson AJ, Gilman RH, Berg DE . Transposable element ISHp608 of Helicobacter pylori: nonrandom geographic distribution, functional organization, and insertion specificity. - J Bacteriol: 2002 Feb, 184(4);992-1002 [PubMed:11807059] [DOI]
- ↑ He S, Corneloup A, Guynet C, Lavatine L, Caumont-Sarcos A, Siguier P, Marty B, Dyda F, Chandler M, Ton Hoang B . The IS200/IS605 Family and "Peel and Paste" Single-strand Transposition Mechanism. - Microbiol Spectr: 2015 Aug, 3(4); [PubMed:26350330] [DOI]
- ↑ Krupovic M, Makarova KS, Forterre P, Prangishvili D, Koonin EV . Casposons: a new superfamily of self-synthesizing DNA transposons at the origin of prokaryotic CRISPR-Cas immunity. - BMC Biol: 2014 May 19, 12;36 [PubMed:24884953] [DOI]
- ↑ Krupovic M, Béguin P, Koonin EV . Casposons: mobile genetic elements that gave rise to the CRISPR-Cas adaptation machinery. - Curr Opin Microbiol: 2017 Aug, 38;36-43 [PubMed:28472712] [DOI]
- ↑ Siguier P, Gourbeyre E, Chandler M . Known knowns, known unknowns and unknown unknowns in prokaryotic transposition. - Curr Opin Microbiol: 2017 Aug, 38;171-180 [PubMed:28683354] [DOI]
- ↑ Hickman AB, Dyda F . Mechanisms of DNA Transposition. - Microbiol Spectr: 2015 Apr, 3(2);MDNA3-0034-2014 [PubMed:26104718] [DOI]