The way in which strand cleavages and transfers occur during transposition also affects the outcome of the transposition events and therefore impinges on genome structure. For IS with DDE Tpases, Tn3 and IS6 family members generate fusions or cointegrates between the donor and target replicons by a process of replicative transposition, presumably by Target Primed Replicative Transposition (TPRT)[1][2][3] (Fig.17.1 and Fig.17.2 top).
However, in the event of intramolecular transposition, this type of mechanism is expected to give rise to inversions with a copy of the IS at each junction or inversions with a single IS copy remaining and a second copy segregating with a circularized deletion[4] (Fig.17.2 botton; see [5]). Note that similar effects are also known to occur by homologous recombination between two inverted or directly repeated IS copies in a replicon. Other known mechanisms such as cut-and-paste, or copy-and-paste (Donor Primed Replicatice Transposition; DPRT)[6] would not generate this type of genomic rearrangement but could contribute to genomic modifications in other ways such as “nearly precise excision”[7] or by using alternative sequences which resemble their IR[8][9].
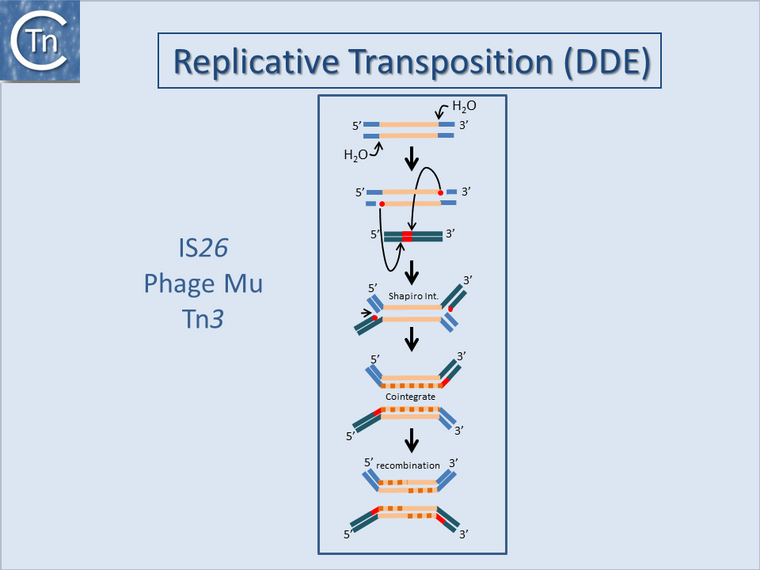
Fig.17.1. Replicative Transposition (
DDE) of TE such as
Tn3 and
bacteriophage Mu. The figure shows the transposition mechanism of replicative TE that uses a DDE Tpase. The transposon is represented as a yellow line. Flanking sequences in the donor molecule is blue. Flanking sequences in the target molecule are green. Red circles indicate 3′OH moieties generated by Tpase-catalyzed hydrolysis at the transposon end(s). Red boxes indicate target DNA flanks that are duplicated on insertion.
Top to bottom: Tpase catalyzed cleavage at the 3′ transposon ends using H2O as the nucleophile. Liberated 3′OH attack the target in a staggered manner to create a branched molecule in which the transposon bridges both donor and target molecules. Replication proceeds, probably using the 3′OH liberated in the flanking target DNA, to generate a second transposon copy (newly replicated DNA is shown as a dotted orange line). If the donor and target DNA are circular molecules, this fuses the two, resulting in a cointegrate where the donor and target DNA are joined at each junction by a TE copy. [Not shown Recombination between the two directly repeated TE ‘resolves’ the cointegrate into the original donor molecule and a target that now contains a copy of the TE. Recombination may use the host homologous recombination system but in the case of the
Tn3 family, TnpR, one of several alternative site-specific recombinases, promotes recombination at a specific DNA sequence, the res site.]
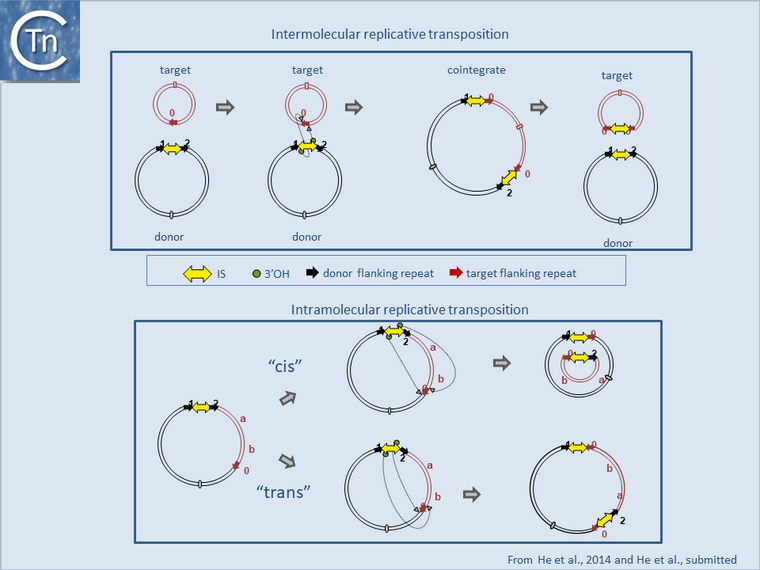
Fig.17.2. Inter- and Intra-molecular replicative transposition.
Top. Inter-molecular transposition. Cleavage at both TIRs of the IS results in nicks on both strands, generating 3-OH groups that attack the target site, leading to the formation of a
Shapiro intermediate. DNA replication generates the cointegrate containing a duplication of the IS and the target site. The cointegrate can be subsequently resolved into a plasmid identical to the original donor plasmid and a modified target plasmid carrying a copy of the IS flanked by TSDs arranged as direct repeats.
Bottom. Intra-molecular transposition. When an IS targets a target site in the same replicon, cleavages at both TIRs generate 3=-OH groups that can either attack the target site on the same strand (cis) or the opposite strand (trans). In the cis pathway, DNA between the IS and target site (dashed lines) becomes circularized and contains one IS copy and target site. In the trans pathway, DNA between IS and the target site is instead inverted (“
a b” becomes “
b a”), bracketed by the original IS, and a new copy in an inverted orientation. The target site is also duplicated but in an inverted orientation, and each TSD is associated with one IS copy.
Bibliography
- ↑
Hickman AB, Chandler M, Dyda F . Integrating prokaryotes and eukaryotes: DNA transposases in light of structure. - Crit Rev Biochem Mol Biol: 2010 Feb, 45(1);50-69 [PubMed:20067338]
[DOI]
- ↑
Hickman AB, Dyda F . Mechanisms of DNA Transposition. - Microbiol Spectr: 2015 Apr, 3(2);MDNA3-0034-2014 [PubMed:26104718]
[DOI]
- ↑
Siguier P, Gourbeyre E, Varani A, Ton-Hoang B, Chandler M . Everyman's Guide to Bacterial Insertion Sequences. - Microbiol Spectr: 2015 Apr, 3(2);MDNA3-0030-2014 [PubMed:26104715]
[DOI]
- ↑
Shapiro JA . Molecular model for the transposition and replication of bacteriophage Mu and other transposable elements. - Proc Natl Acad Sci U S A: 1979 Apr, 76(4);1933-7 [PubMed:287033]
[DOI]
- ↑
He S, Hickman AB, Varani AM, Siguier P, Chandler M, Dekker JP, Dyda F . Insertion Sequence IS26 Reorganizes Plasmids in Clinically Isolated Multidrug-Resistant Bacteria by Replicative Transposition. - mBio: 2015 Jun 9, 6(3);e00762 [PubMed:26060276]
[DOI]
- ↑
Curcio MJ, Derbyshire KM . The outs and ins of transposition: from mu to kangaroo. - Nat Rev Mol Cell Biol: 2003 Nov, 4(11);865-77 [PubMed:14682279]
[DOI]
- ↑
Ross DG, Swan J, Kleckner N . Nearly precise excision: a new type of DNA alteration associated with the translocatable element Tn10. - Cell: 1979 Apr, 16(4);733-8 [PubMed:455447]
[DOI]
- ↑
Ohtsubo E, Zenilman M, Ohtsubo H . Plasmids containing insertion elements are potential transposons. - Proc Natl Acad Sci U S A: 1980 Feb, 77(2);750-4 [PubMed:6244582]
[DOI]
- ↑
Polard P, Seroude L, Fayet O, Prère MF, Chandler M . One-ended insertion of IS911. - J Bacteriol: 1994 Feb, 176(4);1192-6 [PubMed:8106332]
[DOI]
|
|---|
| General Information | Overview, IS History, What Is an IS?, ISfinder and the Growing Number of IS, IS Identification, IS Distribution, Major Groups are Defined by the Type of Transposase They Use, Fuzzy Borders, tIS - IS and relatives with passenger genes, IS derivatives of Tn3 family transposons, IS related to Integrative Conjugative Elements (ICEs), IS91 and ISCR, Non-autonomous IS derivatives, Relationship Between IS and Eukaryotic TE, Impact of IS on Genome Evolution - The Importance of Time Scale, Target Choice, Influence of transposition mechanisms on genome impact, IS and Gene Expression, IS Organization, Control of transposition activity, Transposase expression and activity, Reaction mechanisms, The casposases |
|---|
| Insertion Sequences | IS1 family, IS1595 family, IS3 family, IS481 family, IS1202 family, IS4 and related families, IS5 and related IS1182 families, IS6 family, IS21 family, IS30 family, IS66 family, IS110 family, IS256 family, IS630 family, IS982 family, IS1380 family, ISAs1 family, ISL3 family, ISAzo13 family, IS607 family, IS91-ISCR families, IS200-IS605 family |
|---|
| Transposable Elements | |
|---|

