Difference between revisions of "Transposons families/Tn402 family"
| Line 2: | Line 2: | ||
Tn''402'' was first identified <ref><nowiki><pubmed>321437</pubmed></nowiki></ref> as an insertion of [[wikipedia:Trimethoprim|trimethoprim]] resistance from the IncP plasmid R751 <ref><nowiki><pubmed>4599661</pubmed></nowiki></ref><ref>Jacob A, Shapiro J, Yamamoto L, Smith DI, Cohen SN, Berg D. . Plasmids studied in Escherichia coli and other enteric bacteria. In (ed.),. In: Bukhari AI, Shapiro J, Adhya S, editors. DNA insertion elements, episomes and plasmids . Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.; 1977.</ref> into a [[wikipedia:Lambda_phage|bacteriophage lambda]] derivative phage. Historically, this family of Tn have been intimately linked to integrons and, indeed, Tn''402''-like transposons have proved instrumental in integron dispersal. This first became apparent during the study of integron structures which include an integron integrase gene, ''intI'', and various antibiotic resistance gene cassettes. These structures were found in different sequence environments suggesting that they are mobile genetic elements. Since they did not contain typical transposition-related genes or obvious terminal inverted repeats, it was suggested that integrons were unusual mobile elements distinct from typical transposons <ref><nowiki><pubmed>2560119</pubmed></nowiki></ref>. However, identification of an integron structure which is embedded in the [[Transposons families/Tn3 family|Tn''3'' family]] transposon, Tn''21'', and flanked by 25bp '''I'''nverted '''R'''epeats (IR), in turn flanked by 5bp '''D'''irect '''R'''epeats (DR), suggested that the integron was part of a precursor transposon <ref><nowiki><pubmed>3007931</pubmed></nowiki></ref><ref><nowiki><pubmed>1963947</pubmed></nowiki></ref> whose transposition genes may have decayed following insertion into Tn''21''. | Tn''402'' was first identified <ref><nowiki><pubmed>321437</pubmed></nowiki></ref> as an insertion of [[wikipedia:Trimethoprim|trimethoprim]] resistance from the IncP plasmid R751 <ref><nowiki><pubmed>4599661</pubmed></nowiki></ref><ref>Jacob A, Shapiro J, Yamamoto L, Smith DI, Cohen SN, Berg D. . Plasmids studied in Escherichia coli and other enteric bacteria. In (ed.),. In: Bukhari AI, Shapiro J, Adhya S, editors. DNA insertion elements, episomes and plasmids . Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.; 1977.</ref> into a [[wikipedia:Lambda_phage|bacteriophage lambda]] derivative phage. Historically, this family of Tn have been intimately linked to integrons and, indeed, Tn''402''-like transposons have proved instrumental in integron dispersal. This first became apparent during the study of integron structures which include an integron integrase gene, ''intI'', and various antibiotic resistance gene cassettes. These structures were found in different sequence environments suggesting that they are mobile genetic elements. Since they did not contain typical transposition-related genes or obvious terminal inverted repeats, it was suggested that integrons were unusual mobile elements distinct from typical transposons <ref><nowiki><pubmed>2560119</pubmed></nowiki></ref>. However, identification of an integron structure which is embedded in the [[Transposons families/Tn3 family|Tn''3'' family]] transposon, Tn''21'', and flanked by 25bp '''I'''nverted '''R'''epeats (IR), in turn flanked by 5bp '''D'''irect '''R'''epeats (DR), suggested that the integron was part of a precursor transposon <ref><nowiki><pubmed>3007931</pubmed></nowiki></ref><ref><nowiki><pubmed>1963947</pubmed></nowiki></ref> whose transposition genes may have decayed following insertion into Tn''21''. | ||
| − | Indeed, integrons In0, In2, and In5 were later identified as defective transposon derivatives <ref><nowiki><pubmed>PMC178208</pubmed></nowiki></ref>. This clearly raises the question of how to define and name integrons (see [[Transposons families/Tn402 family#Origin of the Tn402 group integrons.|Integrons]]). We will use a working definition for an integron as a DNA segment composed of an “integron platform” together with a variable number of integron gene cassettes. The integron platform includes an integrase gene, ''intI1'' (a [[wikipedia:Site-specific_recombination|tyrosine site-specific recombinase]]), and a recombination site just upstream (''attI'') at which integron cassettes can be integrated. The cassettes are integrated in an orientation which allows their expression from a promoter, Pc, located within the integrase gene itself (Fig. Tn402.1) | + | Indeed, integrons In0, In2, and In5 were later identified as defective transposon derivatives <ref><nowiki><pubmed>PMC178208</pubmed></nowiki></ref>. This clearly raises the question of how to define and name integrons (see [[Transposons families/Tn402 family#Origin of the Tn402 group integrons.|Integrons]]). We will use a working definition for an integron as a DNA segment composed of an “integron platform” together with a variable number of integron gene cassettes. The integron platform includes an integrase gene, ''intI1'' (a [[wikipedia:Site-specific_recombination|tyrosine site-specific recombinase]]), and a recombination site just upstream (''attI'') at which integron cassettes can be integrated. The cassettes are integrated in an orientation which allows their expression from a promoter, Pc, located within the integrase gene itself (Fig. Tn402.1) <ref><nowiki><pubmed>26104695</pubmed></nowiki></ref><ref><nowiki><pubmed>16845431</pubmed></nowiki></ref>. This definition is restrictive since it does not include the Tn''402'' ends of the transposition enzymes. It raises the important question of how transposable elements such as Tn''402'' and a second transposon family, [[Transposons families/Tn7 family|Tn''7'']], members have acquired these gene cassette-acquiring systems. |
| − | Tn''402'' (U67194.4) is similar if not identical to Tn''5090'' (AM993098) also isolated from | + | Tn''402'' ([https://www.ncbi.nlm.nih.gov/nuccore/U67194.4/ U67194.4]) is similar if not identical to Tn''5090'' ([https://www.ncbi.nlm.nih.gov/nuccore/AM993098 AM993098]) also isolated from R751<ref><nowiki><pubmed>PMC205496</pubmed></nowiki></ref>. Both had subsequently been grouped into a family called Tn''5053'' <ref><nowiki><pubmed>8594337</pubmed></nowiki></ref> although we have elected here to call the family the Tn''402'' family based on its founding transposon. |
| − | More recently, a second group of related transposons, the Tn''Pfu1'' group, was identified which include another integrase gene, ''IntI3'', oriented in the opposite direction to that in the Tn''402'' group | + | More recently, a second group of related transposons, the [[Transposons families/Tn402 family#The TnPfu1 group|Tn''Pfu1'' group]], was identified which include another integrase gene, ''IntI3'', oriented in the opposite direction to that in the [[Transposons families/Tn402 family#The Tn402 group and transposon decay.|Tn''402'' group]] <ref><nowiki><pubmed>PMC6105817</pubmed></nowiki></ref>, and therefore acquired by a Tn''402'' family independently of the type 1 integron, while a third group, [[Transposons families/Tn402 family#The Tn5053 group|Tn''5053'']], include genes for resistance to mercury salts instead of the integron platform <ref><nowiki><pubmed>8387603</pubmed></nowiki></ref>. |
| + | <br /> | ||
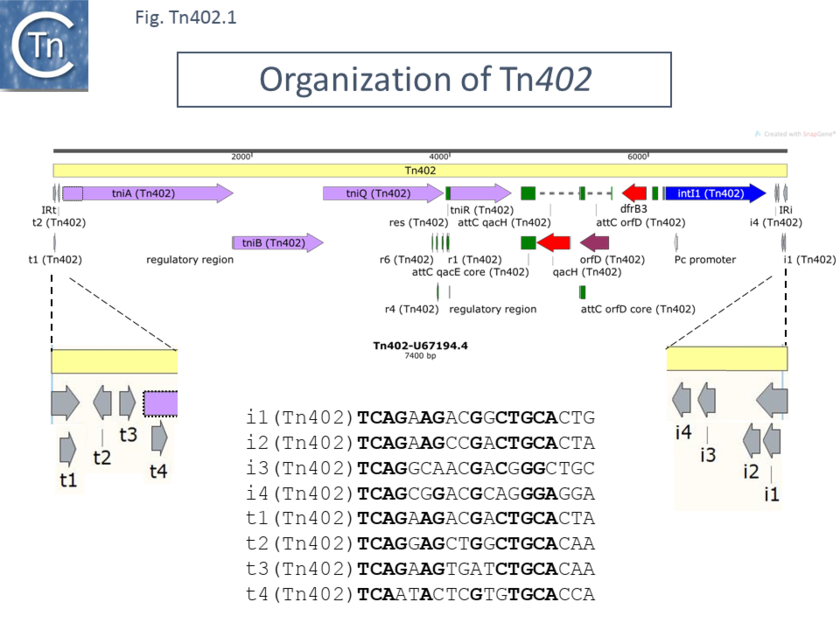
| + | [[File:Fig-Tn402.1.png|center|thumb|840x840px|'''Fig-Tn402.1.''' '''Organization of Tn''402''.''' Tn402 (Genbank accession number: [https://www.ncbi.nlm.nih.gov/nuccore/U67194.4/ U67194.4]) is shown as a pale yellow box. Open reading frames are shown as horizontal boxes where the arrowheads indicate the direction of translation: red, antibiotic resistance genes; lavender, transposase related genes (note that the start codon of TniA may be located further downstream than initially proposed as shown by the dotted outline following comparison with other members of the family – see text); purple, other; blue IntI; green boxes, integron cassette recombination sites and ''res'' sites; the terminal IRs (IRt, transposase proximal; IRi, integrase proximal) are also shown together with the short repeated sub-sites. These are shown in more detail below together with their relative orientations (grey arrows), and nucleotide sequences. The ''res'' site together with its six sub-repeats is shown in green. The figure was generated using [https://www.snapgene.com/ SnapGene].]] | ||
| + | <br /> | ||
====Distribution==== | ====Distribution==== | ||
Tn''402'' family transposons are widely distributed in clinical and environmental isolates. A number of community studies have been carried out including from aquatic environments ��[14]�, permafrost ��[15–18]�, the rhizosphere ��[19,20]� and river and sewage sediments ��[21,22]�. Because of their importance in environmental pollution, there has been much attention given to transposons carrying mercury resistance genes (see ��[23]�). These have been identified, for example, as part of plasmids isolated from the river Mersey (U.K. ;��[14]�) in 1988 where an example was shown to share the same strict target specificity (Insertion Sites: res Hunters) and carry similar mercury resistance genes ��[24]� to Tn''5053'', isolated from a Russian mercury mine ��[13]� in 1984. A derivative, Tn''50580'' carrying a complex set of mercury resistance genes was also identified in samples taken from sediment of the Nura, a highly contaminated river in Kazakhstan ��[22]�. Other examples include, related transposons and more complex Tn''5053'' derivatives in permafrost samples many thousands of years old ��[15]�; variants or transposon fragments with identical restriction maps (or partial sequences) in pre-antibiotic era strains in the Murray collection ��[25]� (''Enterobacteriaceae'' isolated between 1917 and 1954 ��[26]�); association with plasmids also involved with degradation of the herbicide atrazine ��[27]�; and in a variety of bacteria including ''Pseudomonas putida'', ''Escherichia coli'', ''Enterobacter'' and ''Klebsiella'' from water and from soil from various geographical regions mainly, but not exclusively in Russia ��[28]�. | Tn''402'' family transposons are widely distributed in clinical and environmental isolates. A number of community studies have been carried out including from aquatic environments ��[14]�, permafrost ��[15–18]�, the rhizosphere ��[19,20]� and river and sewage sediments ��[21,22]�. Because of their importance in environmental pollution, there has been much attention given to transposons carrying mercury resistance genes (see ��[23]�). These have been identified, for example, as part of plasmids isolated from the river Mersey (U.K. ;��[14]�) in 1988 where an example was shown to share the same strict target specificity (Insertion Sites: res Hunters) and carry similar mercury resistance genes ��[24]� to Tn''5053'', isolated from a Russian mercury mine ��[13]� in 1984. A derivative, Tn''50580'' carrying a complex set of mercury resistance genes was also identified in samples taken from sediment of the Nura, a highly contaminated river in Kazakhstan ��[22]�. Other examples include, related transposons and more complex Tn''5053'' derivatives in permafrost samples many thousands of years old ��[15]�; variants or transposon fragments with identical restriction maps (or partial sequences) in pre-antibiotic era strains in the Murray collection ��[25]� (''Enterobacteriaceae'' isolated between 1917 and 1954 ��[26]�); association with plasmids also involved with degradation of the herbicide atrazine ��[27]�; and in a variety of bacteria including ''Pseudomonas putida'', ''Escherichia coli'', ''Enterobacter'' and ''Klebsiella'' from water and from soil from various geographical regions mainly, but not exclusively in Russia ��[28]�. | ||
Revision as of 19:17, 15 March 2021
Contents
- 1 Historical.
- 2 Distribution
- 3 Organisation
- 3.1 Tn402/Tn5090 Terminal Repeated Sequences
- 3.2 Tn402/Tn5090 Integron Cassettes
- 3.3 Tn402/Tn5090 Transposition-Related Genes
- 3.4 Tn5053 Terminal Repeated Sequences
- 3.5 Tn5053 Functional Analysis of TniR and the res site
- 3.6 TniR Expression and Translation Initiation
- 3.7 TniA Expression and Translation Initiation
- 3.8 TniB Expression, Translation Initiation and Translational Coupling with TniA
- 3.9 TniQ Expression, Translation Initiation and Translational Coupling with TniB
- 4 Diversity.
- 5 The Tn402 group and transposon decay.
- 6 Inter-transposon recombination at the Tn402 res
- 7 Origin of the Tn402 group integrons.
- 8 Insertion Sites: res Hunters.
- 9 A reflection on transposition mechanism.
- 10 Acknowledgements
- 11 Bibliography
Historical.
Tn402 was first identified [1] as an insertion of trimethoprim resistance from the IncP plasmid R751 [2][3] into a bacteriophage lambda derivative phage. Historically, this family of Tn have been intimately linked to integrons and, indeed, Tn402-like transposons have proved instrumental in integron dispersal. This first became apparent during the study of integron structures which include an integron integrase gene, intI, and various antibiotic resistance gene cassettes. These structures were found in different sequence environments suggesting that they are mobile genetic elements. Since they did not contain typical transposition-related genes or obvious terminal inverted repeats, it was suggested that integrons were unusual mobile elements distinct from typical transposons [4]. However, identification of an integron structure which is embedded in the Tn3 family transposon, Tn21, and flanked by 25bp Inverted Repeats (IR), in turn flanked by 5bp Direct Repeats (DR), suggested that the integron was part of a precursor transposon [5][6] whose transposition genes may have decayed following insertion into Tn21.
Indeed, integrons In0, In2, and In5 were later identified as defective transposon derivatives [7]. This clearly raises the question of how to define and name integrons (see Integrons). We will use a working definition for an integron as a DNA segment composed of an “integron platform” together with a variable number of integron gene cassettes. The integron platform includes an integrase gene, intI1 (a tyrosine site-specific recombinase), and a recombination site just upstream (attI) at which integron cassettes can be integrated. The cassettes are integrated in an orientation which allows their expression from a promoter, Pc, located within the integrase gene itself (Fig. Tn402.1) [8][9]. This definition is restrictive since it does not include the Tn402 ends of the transposition enzymes. It raises the important question of how transposable elements such as Tn402 and a second transposon family, Tn7, members have acquired these gene cassette-acquiring systems.
Tn402 (U67194.4) is similar if not identical to Tn5090 (AM993098) also isolated from R751[10]. Both had subsequently been grouped into a family called Tn5053 [11] although we have elected here to call the family the Tn402 family based on its founding transposon.
More recently, a second group of related transposons, the TnPfu1 group, was identified which include another integrase gene, IntI3, oriented in the opposite direction to that in the Tn402 group [12], and therefore acquired by a Tn402 family independently of the type 1 integron, while a third group, Tn5053, include genes for resistance to mercury salts instead of the integron platform [13].
Distribution
Tn402 family transposons are widely distributed in clinical and environmental isolates. A number of community studies have been carried out including from aquatic environments ��[14]�, permafrost ��[15–18]�, the rhizosphere ��[19,20]� and river and sewage sediments ��[21,22]�. Because of their importance in environmental pollution, there has been much attention given to transposons carrying mercury resistance genes (see ��[23]�). These have been identified, for example, as part of plasmids isolated from the river Mersey (U.K. ;��[14]�) in 1988 where an example was shown to share the same strict target specificity (Insertion Sites: res Hunters) and carry similar mercury resistance genes ��[24]� to Tn5053, isolated from a Russian mercury mine ��[13]� in 1984. A derivative, Tn50580 carrying a complex set of mercury resistance genes was also identified in samples taken from sediment of the Nura, a highly contaminated river in Kazakhstan ��[22]�. Other examples include, related transposons and more complex Tn5053 derivatives in permafrost samples many thousands of years old ��[15]�; variants or transposon fragments with identical restriction maps (or partial sequences) in pre-antibiotic era strains in the Murray collection ��[25]� (Enterobacteriaceae isolated between 1917 and 1954 ��[26]�); association with plasmids also involved with degradation of the herbicide atrazine ��[27]�; and in a variety of bacteria including Pseudomonas putida, Escherichia coli, Enterobacter and Klebsiella from water and from soil from various geographical regions mainly, but not exclusively in Russia ��[28]�.
Interestingly, Tn402-like transposons and transposon fragments were identified in a survey of IncP group plasmids identified in the rhizosphere of greenhouse-grown plants ��[19]�. Two included drug resistance gene cassettes but none were associated with mercury resistance. On the other hand, a study of an alfalfa rhizosphere in Germany revealed a number of plasmids carrying mercury resistance among which was pSN102 which carried the Tn402-family transposon Tn5718 ��[20,22]�.
In view of the overuse of antibiotics, it is perhaps not surprising that Tn402 derivatives carrying antibiotic resistance integron cassettes have been found in sewage treatment plants (e.g. TnpRSB105 carried by plasmid pRsB105;��[29]�) and also as rearranged fragments (e.g.��[30–32]�) associated with other (rearranged TE) and also in estuarine waters ��[33]�.
Organisation
Tn402/Tn5090 Terminal Repeated Sequences
Since it is now known that Tn402 and Tn5090 are different isolates of the same element and given that Tn402 was the first described, we have elected to retain the name Tn402 throughout although it should be kept in mind that in some of the literature, the transposon is described as Tn5090. The nucleotide sequence of Tn402 revealed a 7.4 kb region from R751 flanked by two 25bp IRs (Fig. Tn402.1). Two segments of Tn402 including each of the ends were found to be similar to the integron identified in the Tn3 family transposon, Tn21 ��[34]�, and called Tn5092, and to a DNA segment embedded in Tn5086 called Tn5093 ��[10,35]�. In all cases, the IR were flanked by 5 bp direct target repeats different for each Tn. All three Tn included a type I integron integrase intI1 at one end. The IRs are referred to as IRi for the copy proximal to the integrase end and IRt for the copy proximal to the opposite (transposase) end. The transposon ends also include several 19 bp repeats: t1, t2 and t3 located at the IRt end with t2 oriented inversely and i1, i2, i3 and i4 located at the IRi transposon end (Fig. Tn402.1). A fourth repeat, t4, can be identified by inspection. These are binding sites for the transposase ��[36]� and their differential organisation at each end would serve to distinguish between the ends in the transposition process as has been shown for transposon Tn7 ��[37–39]� and bacteriophage Mu ��[40,41]� (Fig. Tn402.2) and members of the IS21 family ��[42]�.
Tn402/Tn5090 Integron Cassettes
Tn402 carries three integron cassettes. One of these contains the trimethoprim resistance gene, dhfrIIc (dihydrofolate reductase type IIc) originally identified in the first transposition experiments ��[1]�. This is located proximal to the integron platform promoter (Fig. Tn402.1). Another, orfD, of unkown function but observed in other integrons, is located downstream and this is followed by a gene encoding the exporter, qacE/H.
Tn402/Tn5090 Transposition-Related Genes
Four transposition-related orfs were also identified. Three of these, tniA, tniB and orf6, now called tniQ, ��[11]� specify proteins of 559, 302 and 405 amino acids respectively. They occur in that order and are all oriented in the same direction (Fig. Tn402.1). They probably constitute an operon expressed from a predicted single promoter ��[10]�. The fourth gene, initially called tniC, but now renamed as tniR (see below) encodes a 207 amino acid protein.
Tn402/Tn5090 Transposition-Related Genes: tniA
TniA was predicted to be a transposase since it shows some weak similarity to the transposon Tn7 transposase TnsB (Fig.Tn402.2), and to the Tn552 transposase (25% identity) and includes a DDE motif typical of the catalytic site of many transposases. tniA is located close to IRt. Transposon Tn7, TnsB, which binds to the multiple short 22bp repeated sequences located in the left and right Tn7 ends (Fig.Tn402.3), suggesting that TniA may bind to the 19 bp repeated sequences in the Tn402 ends. This has now been demonstrated using DNase and hydroxy-radical footprinting approaches ��[36]�. It should be noted that there is some ambiguity in the position of the TniA initiation codon (see below; Fig. Tn402.6).
Tn402/Tn5090 Transposition-Related Genes: tniB
The product of the second orf, TniB (Fig. Tn402.1), contains predicted nucleotide triphosphate binding sites and was proposed to be involved in nucleotide triphosphate hydrolysis ��[10]�. It exhibits 21% identity to the Tn7 TnsC protein, an ATPase ��[43]� which binds non-specifically to DNA in the presence of ATP ��[44]�, and is required for transposition. TnsC also binds to the TnsB component the heterodimeric TnsA/TnsB transposase and couples it to a protein involved in targeting Tn7 insertion, TnsD ��[45]�. TniB also shows 24% identity to the MuB protein, also a DNA-dependent ATPase that preferentially stimulates intermolecular DNA strand transfer in bacteriophage Mu ��[46]�.
Tn402/Tn5090 Transposition-Related Genes: tniQ
The third gene (Fig. Tn402.1) was called orf6 ��[10]� but has since been named tniQ ��[11]� and its product did not exhibit similarity to known proteins ��[10]�. It is positioned such that its initiation codon overlaps the termination codon of the upstream tniB gene (see below; Fig. Tn402. 7) indicating that expression of the two gene products is translationally coupled.
Tn402/Tn5090 Transposition-Related Genes: tniR
The product of the fourth gene belongs to the invertase/resolvase family of site-specific recombinases. It has significant identity (67%) to the resolvase of the Tn3 family transposon, Tn5393, 47% identity to the Gin recombinase of phage Mu ��[47]�. For this reason, it is now generally referred to as tniR for Resolvase. It is probably expressed separately from the other three genes and appears to have its own promoter ��[10]� (see below; Fig. Tn402.5).
Tn5053 Terminal Repeated Sequences
More information was obtained from the related Tn5053 (L40585.1), a mercury resistance transposon isolated from the chromosome of a mercury resistant Xanthomonad from a mercury mine ��[13]�. Tn5053 carries a set of transposition genes which are very similar to those of Tn402/Tn5090. Like other members of the family it is bordered by 25 bp inverted repeats (Fig. Tn402.4) and includes short ~19 bp repeat sequences at its ends. Careful inspection of the Tn5053 sequence identifies additional 19bp repeat sequences than those reported ��[13]�, as in the case of Tn402/Tn5090 (Fig. 402.1). In this case there are 4 copies at the IRt end and three at the equivalent of the IRi end. Also, in the original article, it appears that those at the IRt and IRi ends have been exchanged compared to Tn402/Tn5090. Tn5053 carries a full complement of transposition genes tniA,B,Q and R but, instead of the integron recombination platform and antibiotic resistance gene cassettes, the transposon carries a mercury resistance operon (Fig. Tn402.4).
Tn5053 Functional analysis of Transposition-Related Genes
Transposition of a collection of insertion and deletion mutants of Tn5053 introduced into tniA, tniB and tniQ was tested in a conjugation (mating out ��[48]�) assay using an RP4 plasmid derivative as a target ��[11]�. Transposition was undetectable in all mutants but could be complemented by supplying a set of Tn5053 genes in trans from a third compatible plasmid. It was also shown that the set of three genes from Tn402 were capable of complementing the mutant Tn5053 derivatives ��[11]�. To address whether the mutations may have a polar effect on the downstream genes, mutations in the complementing set of genes were used: a Tn5053 tniA mutant could be complemented by a tniA operon with a wildtype tniA and carrying mutations in tniB and tniQ , while a tniB mutant could be complemented by a tniQ/tniR mutant. It is unclear whether a tniB mutant could be complemented by a tniA/tniQ/tniR mutant raising the formal possibility that some polarity effects may occur.
Tn5053 Functional Analysis of TniR and the res site
As might be expected from the similarity of tniR to site-specific resolvases, Tn5053 appears to transpose via a cointegrate intermediate. Ablation of the tniR gene and part of the tniQ gene resulted in a transposon unable to undergo transposition, presumably because of the defective TniQ. Complementation using the entire set of transposition genes resulted in high transposition levels. In the absence of TniR in the complementing plasmid, cointegrates carrying both donor and recipient plasmid antibiotic resistance markers were identified. Resolution required the presence of only TniR and not TniA, B or Q. The recombination site was located using a system with cloned Tn restriction fragments from a region upstream of tniR and the “kinetics“ of resolution was followed. It was concluded that a region close to the 5’ end of the tniR gene which, on its own resulted in slow resolution, represented the principal resolution site while a region stretching upstream into the 3’ end of the tniQ gene was required for robust activity and therefore presumably carried secondary stimulating sequences. This region includes six 14 bp repeat sequences with the consensus PyTGTCACPuT(NT)(NT)NC(C/G), labelled r1-r6 (shown in detail in Fig. Tn402.5A and B). Most site-specific resolution systems include multiple short repeated sequences which serve as recombinase binding sites permitting the assembly of architecturally precise nucleoprotein complexes to tightly control recombination. The repeats include r1 and r2 which are inverted, almost abut the start of the tniR gene and are separated by a dinucleotide, AA, proposed as the crossover point in the resolution recombination reaction (Fig. Tn402.4B). Additional copies are observed further upstream and located within the 3’ end of tniQ (Fig. Tn402.5A and B) suggesting that TniQ expression and resolution are intimately coupled. Further analysis is necessary to provide direct evidence that these repeated sequences are TniR binding sites and to establish a detailed recombination mechanism.
TniR Expression and Translation Initiation
A potential promoter ��[11]� for tniR expression is also located in this region (Fig. Tn402.5B) and is clearly well placed to be regulated by TniR binding. Fig. 402.5B also shows that the equivalent region of Tn402/Tn5090 is very similar with related repeat sequences and potential promoter elements. However, there is additional G residue and changes at the potential TniR ATG codon compared to those observed in Tn5053. However, there is a suitably placed in phase ATG codon slightly further downstream in both Tn402/Tn5090 and in Tn5053 which suggests that this maybe the true initiation codon for TniR.
TniA Expression and Translation Initiation
In addition to the ambiguity of the TniR initiation codon, a similar question arises concerning identification of the initiation codon of TniA (Fig. Tn402.6). Certain family members (e.g. Tn402 and integron members) have an orf with an initiation codon upstream of the final t repeat (Fig. Tn402.6) whereas others (e.g. Tn502 and Tn512) appear to initiate further downstream. Alignment of the DNA sequences shows that all members are very similar in sequence in this region but that those with the downstream start codon each contain a 2 bp insertion which places the upstream region out of phase (Fig. Tn402.6). Since Tn502 and Tn512 and Tn5053 are fully functional ��[13,49,50]�, it seems possible that it is the downstream start codon that is used in all family members (Tn402.9 TniA). But this will need experimental confirmation.
TniB Expression, Translation Initiation and Translational Coupling with TniA
A similar ambiguity arises with TniB. A DNA alignment (Fig. Tn402.7A), however, not only shows a high degree of identity but suggests that there may be some form of translational coupling in the expression of TniA and TniB. There are potential TniB GTG start codons 1bp upstream and 2bp downstream of the predicted TniA termination codon (TAG) ��[51]�. Although we have chosen to use the downstream GTG (Fig. Tn402.9 TniB), it should be remembered that if the upstream GTG is used, TniB would include an N-terminal extension of three amino acids, MVA. Note that the 2 mutations shown in the figure are synonymous (ATT/ATC) and conservative (GAC/GAA) respectively.
TniQ Expression, Translation Initiation and Translational Coupling with TniB
For many examples of Tn402 family transposons, the initation codon of TniQ (GTG) overlaps the termination codon (TGA) of TniB (Fig. Tn402.7B), a situation typical of translational coupling ��[51]�. All members of the family so far identified show high conservation and all contain the overlapping GTGA initiation/termination codons. Although some family members had included annotated TniB and or TniQ genes which had internal start codons, the most parsimonious interpretation is that the entire family follow identical initiation and termination rules (Fig. Tn402.9 TniQ).
Diversity.
There are three principal groups which constitute the Tn402 family based on the type of passenger genes they carry. The first, Tn402, includes those which have acquired an integron recombination platform of type 1 with an integrase IntI1 derivative. The second, TnPfu1, is a small group composed of members which have integrated a type 3 integron recombination platform with an IntI3 integrase. The third group, Tn5053, includes members which do not carry integron function but instead carry mercury resistance genes.
A phylogenetic tree of the concatenated TniA,B,Q and R proteins (Fig. Tn402.8) indicated that Tn5053 group members carrying mercury resistance genes tend to be clustered with Tn502 and Tn512 although several are intermingled with Tn402 IntI1 carrying transposons. This is perhaps not surprising since many Tn5053 members were selected using a BLAST search. A similar argument can be proposed for the three TnPfu1 related transposons which carry an IntI3 gene.
When considered individually, the different gene products are very closely related: TniA (Fig. Tn402.8A; XXX-YYY identity, ZZZ-AAA similar); TniB (Fig. Tn402.8B; XXX-YYY identity, ZZZ-AAA similar); TniQ ((Fig. Tn402.8C; XXX-YYY identity, ZZZ-AAA similar) and TniR (Fig. Tn402.8D; XXX-YYY identity, ZZZ-AAA similar).
The fact that the TniA,B,Q and R proteins are highly conserved (Fig. Tn402.9) whereas the DNA sequences are more variable (see Fig. Tn402.11 and Fig. Tn402.13) (and therefore the DNA variability mainly introduces synonymous mutations together with some conservative amino acid changes; Fig. Tn402.9) suggests either that the phylogenetic branches are quite recent or that there is significant selective pressure on the protein ensemble.
The major differences between each of the Tn402 family members lie in the passenger genes which they carry.
The Tn402 group and transposon decay.
As stated above, there has been some ambiguity in the literature concerning the nomenclature of Tn402 family members as transposons or as integrons and that this has been due to decay in the Tn402 tniABQR transposition module. In this group, the antibiotic resistance genes are oriented towards and downstream of the transposition genes (Fig. Tn402.1). Fig. Tn402.10 shows an alignment of an illustrative group of Tn402 elements. All the examples include the right (integrase proximal) Tn402 end, IRi and its 19 bp repeated sequences together with the integron integrase, intI1 gene, the binding site for the host LexA protein which couples IntI1 expression to the host SOS system ��[8,52,53]�, the integron cassette promoter Pc which drives expression of the genes carried in the integron cassettes, and the attI recombination site which serves in the acquisition of integrons cassettes ��[8]�. This is followed by the integron cassettes which vary from element to element and are indicated by the small arrow heads. Tn402 (U67194.4) itself carries three cassettes: qacH; orfD, a gene of unknown function; and the dihydrofolate reductase gene, dfrB3. The IS402 family member with the lowest number of integron cassettes is In_Tn1721.1 (HQ730118.1) which includes only qacH and its recombination sites while Tn402.5 (MH392234) ��[19]� carries, in addition, a second cassette with a gene of unknown function.
Within this group, Tn402.5 (MH392234), In_Tn1721.1, Tn402.4 (MW150990), Tn6112,(HQ423158 ) ��[54]�, TnpHS87a (KR106190) ��[55]�, and TnXorYNA12 (HQ662557) ��[56]� all carry a full complement of the transposition module and a variety of different integron cassettes.
A second group has lost both tniR and tniQ genes. These include: In0 (U49101) ��[57]� where they, together with the 3’ end of tniB, have been replaced by an insertion of IS1326 upstream of a GNAT cassette; Tn7017 (NC_022242.1) ��[58]� in which the In0 GNAT cassette abuts a further 3’ deleted tniB (presumably following excision of the IS1326 copy); TnpRSB105 (DQ839391) ��[29]� which has a similar tniB-GNAT junction but in which a tip of IS1326 (GGCCTG) has been retained; In2 (AF071413) which contains an insertion of IS1353 into the istA gene of IS1326, a common feature of a number of integron structures; In_Tn4 (KY749247.1) ��[59]� which, except for the absence of the IS1353 insertion, is identical to In2; In_TnAs3 (CP000645) which has an identical tniB-GNAT junction as Tn7017; In34 (AY123253 ) ��[60]� is complex and, in addition, to carrying the IS1326::IS1353 insertion, also carries an insertion of the IS26-based transposon Tn4352 in tniA; In_Tn1935 (MK797990) is identical to In34 except for its integron cassettes; and, finally, In_Tn5045 (FN821089) ��[16]� carries the tniB-GNAT junction and also a Tn3-family chromate resistance transposon, Tn TnOtChr.1, at the 5’ end of tniB. For In31 (AJ223604) ��[61]�, tniB is truncated further to its 5’ end than those with a tniB-GNAT junction and for In_Tn21.1 (MH257753) an IS26 insertion has led to a 3’ truncation of the tniA gene.
A third group, which includes In33 (AF313471) ��[62]�, In36 (AY259085) ��[63]�, In4 (U12338.3) ��[64]�, In37 (AY259086) ��[63]� and In_Tn6025 (GU562437) have all undergone a complex rearrangement involving IS6100 and duplication of IRt and repeats in an inverted orientation surrounding the IS6100 copy.
The fourth group, including In7 (L06418)��[65]�, In6_p (L06822) and InS21 are closely related but are only partially sequenced examples. They do not include an IRt end and include an insertion of ISCR1 (IS99 and ISCR family). However, the reported partial integron In7 (KU997026) from Escherichia coli plasmid pDGO100 ��[66]� closely resembles In34. It extends both upstream to include the first IS26 copy of transposon Tn4352 inserted in tniA of In34 and downstream to include the IRi end. The In7 (L06418) copy ��[65]� is a sub-sequence of this. A similar problem is likely to arise with In6_p (L06822) ��[67]� which also shares several identical regions with In34. In6_p includes a full set of IRi sequences downstream of its IntI1 gene but the sequence of its pSa plasmid parent ��[68]� is not available. Finally, InS21 (AJ311891), again with significant regions identical to In34, from plasmid pS21, carries a complete IRi end but is truncated at the opposite end.
There is consequently no strong evidence that integron platforms along with their cassettes have been transferred without their accompanying Tn402 transposition system. “Integron” insertion may have occurred prior to decay of the tniABQR genes (see ��[21]�).
Inter-transposon recombination at the Tn402 res
As in other transposons which use a site-specific resolution mechanism as a step in their transposition ��[69,70]�, members of the IS402 family can also undergo inter-transposon recombination at the res site and in this way exchange DNA modules. This type of behavior has been reported by Labbate et al. ��[70,71]� who observed that transposon Tn6007 from Enterobacter cloacae included a class 1 integron with two non-antibiotic-resistance-type gene cassettes and a complete transposition module. The tniABQR module appeared to be a hybrid with a boundary within the res site (Fig. Tn402.11). The level of identity from the res site through tniR and the integron was observed to be high (99.9%) while that further upstream through the tniA,B and Q genes was only 89% ��[71]�. This is not an isolated case and a number of additional examples of this phenomenon can also be observed (Fig. Tn402.11). Similar observations were made by Betteridge et al. in an analysis of integron platforms isolated from Human Commensal Bacteria in an intensive care unit (ICU) ��[72]�.
Origin of the Tn402 group integrons.
One hypothesis to explain the origin of the mobilizable class 1 integron is that it is derived from a chromosomal integron platform that became mobile by the acquisition of Tn402-like transposition functions. However, the identification of two other Tn402 family groups with totally different passenger gene ensembles tends to suggest that it might have been an ancestral Tn402 transposon which acquired the passengers.
The suggestion that some class 1 integrons identified in a sediment microbial community potentially predate integron association with Tn402 is difficult to assess ��[21]�. Also, no ancestral Tn402 transposon has yet been identified without passenger genes.
The TnPfu1 group
In the majority of Tn402 family transposons, integron platform is of type 1 and the integrase gene is known as intI1. IntI1 is invariably located at the distal transposon end and is expressed in the same direction as tniA, however, there is an additional group in which the integrase faces towards tniR.
An example of a Tn402 derivative with this configuration of tniA and intI genes, TnPfu1 (LC331665.1), (Fig. Tn402.11) was first identified from Pseudomonas fulva and carried a blaIMP-1 gene together with AAC(6') and fosX gene cassettes ��[12]�. A BLAST search for tniA -intI1 regions with this configuration revealed only two additional examples, all closely related except for the gene cassettes carried by the integron platform: TnPfu1.1 (LC589064) carries a bla GES gene and a cassette containing AAC(6')-Ib8/-Ib4, while TnPfu1.2 (LC589062) includes two copies of bla GES.
Members of this group carry another type of intI gene associated with Tn402-like transposition genes, intI3. This was first identified in Serratia marcescens plasmid pSMB731 as part of a new integron type, type 3 ��[73]�, in which the integrase showed only limited identity to IntI1. Unfortunately, only partial sequence data was presented (Fig. Tn402.12) although a limited extension was published more recently ��[74]� covering the tniA gene upstream and an AAC(6')-Ib8 gene cassette downstream together with the IRi end (Fig. Tn402.12).
The Tn5053 group
Tn5053 (L40585.1) itself (Fig. Tn402.4), isolated from a Russian mercury mine ��[13]�, carries an operon specifying resistance to mercury. The mercury resistance genes, like the antibiotic resistance genes in the Tn402 group, are oriented such that expression occurs towards and downstream of the transposition genes. For the sake of simplicity, the IR distal to the transposition genes has retained its name from Tn402, IRi.
As for the Tn402 and TnPfu1 groups, the TniA,B,Q and R proteins are well conserved (Fig.Tn402.9) while the DNA sequences show a higher degree of variation (Fig. Tn402.13).
Inter-transposon recombination at the Tn5053 res
Inter-transposon recombination at the res site is also observed in the case of family members which carry mercury resistance genes in place of the integron platform. This can be seen between Tn5053 (L40585.1) and a related mercury resistance transposon we have called Tn5053.1 (CP002451) from the Alicycliphilus denitrificans BC plasmid pALIDE02 ��[75]� (Fig. Tn402.14) where the tniABQ module has clearly been exchanged . This is would at least partially explain why Tn5053 and Tn5053.1 are located in different subgroups in the phylogenetic tree (Fig. Tn402.8)
Presence of a Tn21-like IR
The relationship between members of this mercury resistance Tn402 group is complex. One close relative, Tn5053.4 (CP001919), carries a simple insertion of IS4321 at the distal end.
Interestingly, all mercury resistance Tn402 family members carry a sequence resembling a Tn21 IR, suggesting that the mercury resistance genes may have be acquired from a Tn21-derivative transposon towards the IRi end (Fig. Tn402. 15) , suggesting that mercury resistance was originally acquired from a Tn21-related Tn3 family transposon (see ��[76]�). However, Tn5053.2 (CP024682) does not and is also missing the MerA sequence up to and including merR due to insertion of an ahp gene Moreover, in a subgroup, there has been a partial duplication of the mercury resistance operon followed by some decay and rearrangement with changes in gene number and order. This may have involved the Tn21-like IR since these transposons, Tn5053.6 (JX469833), Tn5053.8 (CP017991), Tn5053.9 (CP048650), Tn5058 (Y17897) and Tn50580 (AM048832), all carry two Tn21-like IR.
Tn5053 group Mercury resistance genes.
Mercury resistance genes are generally grouped into an operon. The major players are: merA, encoding a mercuric reductase (MerA), which catalyses reduction of Hg(II) to volatile Hg(0) and is the central enzyme in Hg(II) resistance ��[77,78]�; merB encoding an organomercury lyase (MerB); merP encoding a periplasmic Hg(II) scavenging protein (MerP); merT, merC, merE, merF and merG which encode inner membrane spanning proteins (MerT, MerC, MerE, MerF, MerG) that transport Hg(II) to the cytoplasm where it is reduced by MerA; merR and merD encoding the regulatory proteins (MerR, MerD). MerR is a transcriptional repressor in the absence of Hg(II) and an activator in its presence and MerD downregulates the operon (for review see ��[79]�) .
Mercury resistance operons do not all carry a complete set of these genes. Fig. Tn402.16 shows a sample of mercury resistance operons from various transposons of the Tn3 and Tn402 families together with the ensemble of mer genes in the order in which they occur in the canonical Tn3 family transposon Tn21.
Insertion Sites: res Hunters.
In early studies describing Tn502 and its differences with other mercury resistance transposons known at the time ��[80]� it was observed that it displays a preference for insertion into a single PstI fragment of the plasmid RP1 ��[49]�. Further restriction mapping led to the conclusion that the insertions occurred at the same site, largely in one orientation. Deletion of this region resulted in a marked reduction in Tn502 insertion frequency into the plasmid ��[80]�. This region was subsequently shown to carry the non-transcribed spacer of the par locus of plasmid RP1, initially defined in the related plasmids RK2 and RP4 as a region required for plasmid stabilisation ��[81,82]�. It is similarly organised to the res region of Tn3 family transposons (see ��[69,76]�) and contains three binding sites for the ParA site-specific recombinase (resI, resII and resIII )��[83]�.
A similar sequence-specific insertion of Tn402/Tn5090 into derivatives of plasmids R388 and RP1 was also demonstrated experimentally ��[84]�. In RP1, insertions occurred in two major clusters (Fig. Tn402.17A) within 140 bp: one cluster (1) was located in the resII resolvase subsite and the second (2) within the parC gene. The orientation of Tn402 insertions in each cluster was inverted. A single major insertion site was observed in plasmid R388, again, close to the proposed res sites ��[84]�. Furthermore, this targeting required an intact ParA since partial deletion significantly reduced the attractiveness of the derivative plasmid as a target. Moreover, a plasmid in which the ParA active site serine was mutated completely retained its activity as a transposition target ��[84]� showing that it is the binding of resolvase and presumably the architecture of the res-ParA nucleoprotein complex rather than recombination activity which determine target activity.
Tn2521, a multi-resistant transposon identified in the chromosome of Pseudomonas aeruginosa clinical isolates in 1982 ��[85]� was also observed to have marked integration site-specificity in transposition into IncP plasmids (in this case R18-1, identical to R68). Tn2521 was later shown to be an integron (In33) without tni genes and its insertion point in the R18-18::Tn2521 example examined was shown to have inserted at the R18-18 equivalent to RP1/RP4 site resII ��[62]�. Moreover, additional analysis of the flanking sequences of other integron structures indicated that insertion occurred in the res sites of several Tn3 family transposons ��[86]�: In28 in the resI region of Tn1403; In4 abutting resI in Tn1696; In1 upstream of resP in R46 ( ��[87]�, also called TP120 ��[88]�), the region expected to include the plasmid res site which has an adjacent functional resolvase-like gene, uvp1 ��[89]�, and is similar to and can undergo recombination with that of Tn1 ��[90–92]�. In these examples, the inserted integron platform does not contain the set of Tn402 transposition enzymes. This implies either that transposition preceded decay of the transposition genes or that their products were provided in trans from another transposon in the cell as is suggested by transposition of In33 (Tn2521). A further example, however, is the insertion into Tn1721 of an integron with a full complement of Tn402 transposition enzymes (HQ730118) ��[72]�.
This type of insertion site-specificity was also observed for Tn5053 ��[13]�, again using RP1 and an RP4 derivative plasmid target. Sequence analysis showed that the insertions were flanked by a 5 bp direct target repeat as expected ��[13,24]�. A survey of conjugative plasmids (cited in ��[93]�) identified only a single example which acted as an efficient Tn5053 target. This plasmid carried Tn701, a close relative of Tn1721 and The Tn5053 insertion were reported to have occurred in the res site of this transposon. Introduction of Tn701 or Tn1721 into plasmids not generally active targets such as R387, pOXGen or pBR322, these became efficient Tn5053 targets ��[93]�. In addition, a database search using the first 50 bp of the ends of Tn5053 and Tn402 as the query sequences revealed a number of Tn junctions in which the IRi ends were located upstream of resolvase-like genes ��[93]�.
In these studies ��[93]�, Insertion of Tn5035 was observed experimentally using as target the cloned insertion-free res site of Tn5044 ��[94]� together with the native TnpR gene. Very strong clustering was observed between resI and resII and all but one insertion occurred in the same orientation (FigTn402.17C). Moreover, when cloned and in the absence of TnpR, the res sites of Tn1721 ��[95]�, Tn5036 ��[18]�, Tn5051 (no sequence available) ��[18,93]� and Tn5059 (Y10102, partial sequence) were not attractive to Tn5035 insertion, however, when TnpR from Tn1721 was supplied in trans, efficient targeted insertion occurred and sequence analysis showed strong clustering (Fig. Tn402.17D). This behavior rules out the possibility that expression (transcription or translation) of TnpR in its natural proximity to res provides a suitable integration substrate. A similar requirement for the ParA resolvase was reported for insertion into the RP1 res. Surprisingly target attractiveness appeared to be limited to the Tn21 branch of the Tn3 family ��[6,96]� neither Tn1 nor Tn1000 were found to be efficient targets for Tn5053 insertion.
Tn402 family members with mercury resistance genes, Tn502 and Tn512, have also been found to show quasi-identical insertion specificity in an RP1 res target ��[50]� as Tn402 itself (Fig. Tn402.17B). Remarkably, the insertion site observed in resII was identical to that observed for Tn402/Tn5090 (cluster 1 in Fig. Tn402.17A) ��[84]�. The authors also address the potential mobility of integron platforms, such as In0 (U49101) and In2 (AF071413) which do not contain a full set of Tn402 tni genes but carry both IRi and IRt ends and are found with flanking 5 bp direct target repeats. Transposition of In2 from its location in Tn21 and of In0 from its location in plasmid pVS1 was tested by supplying Tn502 tni genes in trans. Transposition occurred at significant frequencies if both tni and the RP1 par site were present. Moreover, the vast majority of insertions occurred in the expected orientation at the cluster 1 site (Fig. Tn402.17B). One puzzling observation was that although neither In0 nor In2 include a Tn402 res site never-the-less, the transposition products had undergone resolution.
Complementation by a resident Tn402 family transposon would provide an explanation for transposition of Tn2521 (In33) into the R18-1 ��[85]� res site even though it does not contain a single Tn402 transposition gene ��[62]�.
In summary, all members of the Tn402 family tested appear to target res sites of plasmids and Tn3 family transposons of the Tn21 group. They require the cognate resolvase protein ��[84,93]� but do not require it to be catalytically active ��[84]�. The role of TnpR could be structural, providing an architecturally precise nucleoprotein complex for integration and/or furnishing an interface for direct protein-protein interaction with a TniABQ component. Transposons with tni gene sets can also complement in trans integron platforms which are lacking these genes ��[50]� in principal providing the capacity to mobilise non-autonomous integron structures.
A reflection on transposition mechanism.
Except for the bioinformatic analysis of their transposition proteins and the demonstration that their transposition requires a cointegration step which is resolved at a specific site, the res site and involves the TniR site-specific recombinase ��[11]�, there has been no significant biochemical or structural studies of Tn402 family transposition.
Studies with purified TniA ��[36]� have shown that the protein binds to the left IRt end of Tn402/Th5090 to generate six retarded bands (I-VI). This was further explored using DNase footprinting which showed that the four sites t1-t4 were Species I-III
or of the resolvase TniR to the repeated sequences at the res site. It is therefore not known which of the multiple end sites are important for transposition or whether all repeated sequences in and around the proposed crossover point between r1 and r2 are necessary for resolution.
Neither have the roles played by TniA, TniB or TniQ been determined. The fact that TniA carries a DDE motif and shows 25% similarity to TnsB transposase ��[97]� of transposon Tn7 ��[10,11]� strongly suggests that it is the Tn402 family transposase and the observation that TniB shares similarities with TnsC suggests that it may play a DNA-dependent bridging reaction between TniA and TniQ. It seems possible that, like TnsD and TnsE of Tn7 ��[97–99]�, tniQ is involved in the choice of the target sequence. Binding to the small repeated sequences at IRt and IRi also has yet to be demonstrated.
In view of the sequence similarities between the Tn7 and Tn402 family transposition proteins, it is interesting to note that the Tn402 family possesses a dedicated system to resolve cointegrates whereas Tn7 does not. In contrast, Tn7 encodes two endonucleases, TnsA and TnsB. The DDE transposase, TnsB, binds to the Tn7 ends cleaves only one strand of the transposon whereas TnsA, which resembles a restriction endonuclease ��[100]�, cleaves the complementary strand. Together, both, form a heteromeric complex and in concert, achieve cleavage and joining reactions at both Tn7 ends ��[101]�. Transposition of Tn7 normally occurs by a cut-and-paste mechanism ��[102]�. However, although TnsA is required for TnsB activity, mutation of the active site of TnsA, prevents the cut-and-paste pathway and results in the formation of cointegrates ��[103]�. Therefore, it seems probable that TniA, like TnsB, catalyses only single-strand cleavage at each transposon end and to deal with the resulting cointegrate structures, Tn402 has recruited a resolvase-like protein and an accompanying abutting res site while Tn7 has recruited a second restriction endonuclease protein.
An additional observation is that the majority of Tn402-family transposons and integrons are flanked by 5 bp direct target repeats. This implies that intermolecular transposition represents a significant, if not unique pathway since intramolecular transposition using a cointegrate pathway leads to loss of the direct target repeats (Fig.17.1 and Fig.17.2)
Acknowledgements
We would like to thank Steve Petrovski (La Trobe University, Australia) for critical comments.
Bibliography
- ↑ <pubmed>321437</pubmed>
- ↑ <pubmed>4599661</pubmed>
- ↑ Jacob A, Shapiro J, Yamamoto L, Smith DI, Cohen SN, Berg D. . Plasmids studied in Escherichia coli and other enteric bacteria. In (ed.),. In: Bukhari AI, Shapiro J, Adhya S, editors. DNA insertion elements, episomes and plasmids . Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.; 1977.
- ↑ <pubmed>2560119</pubmed>
- ↑ <pubmed>3007931</pubmed>
- ↑ <pubmed>1963947</pubmed>
- ↑ <pubmed>PMC178208</pubmed>
- ↑ <pubmed>26104695</pubmed>
- ↑ <pubmed>16845431</pubmed>
- ↑ <pubmed>PMC205496</pubmed>
- ↑ <pubmed>8594337</pubmed>
- ↑ <pubmed>PMC6105817</pubmed>
- ↑ <pubmed>8387603</pubmed>