Difference between revisions of "IS Families/IS30 family"
(→IS30) |
(→ISApl1) |
||
| Line 31: | Line 31: | ||
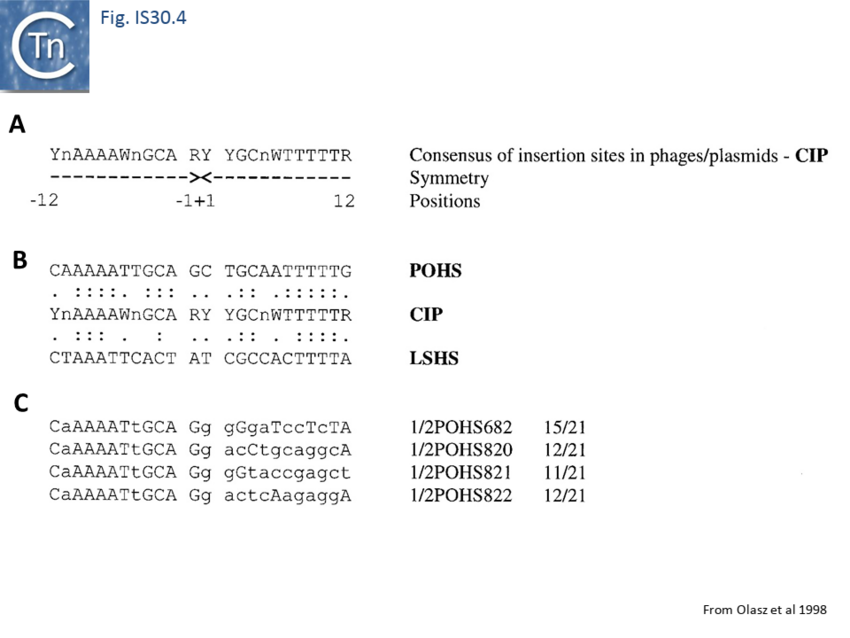
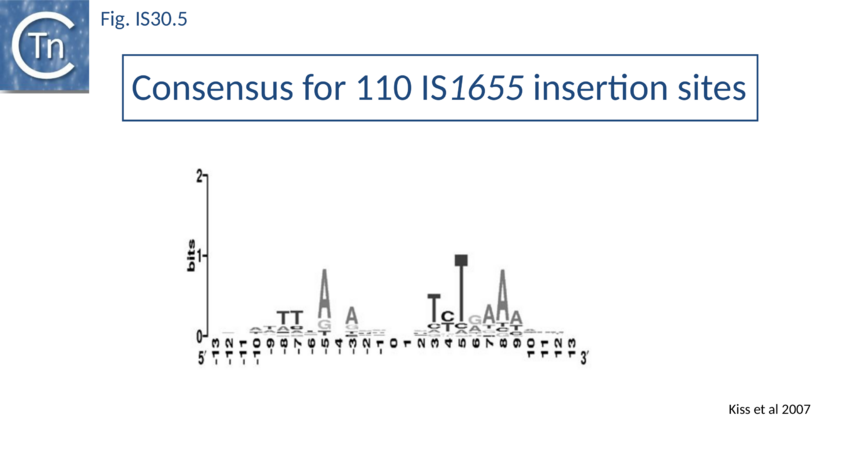
Like IS21<ref><nowiki><pubmed>3034717</pubmed></nowiki></ref> and IS911<ref><nowiki><pubmed>10760133</pubmed></nowiki></ref>, IS30 shows a preference for insertion next to nucleotide sequences resembling its ends<ref><nowiki><pubmed>9393723</pubmed></nowiki></ref>. Furthermore, this region has been delimited approximately to the Tpase binding site. In addition, preferential insertion into a site in bacteriophage l (LHS) and P1 (OHS) has been observed both in E.coli<ref><nowiki><pubmed>8389976</pubmed></nowiki></ref> and Salmonella<ref><nowiki><pubmed>10570999</pubmed></nowiki></ref>. These sequences are somewhat degenerate palindromes characterized by a 24 bp symmetric consensus<ref><nowiki><pubmed>9643538</pubmed></nowiki></ref> (Fig. IS30.4). The sequence relationship between these various hotspots has yet to be explored. A similar type of insertion specificity was observed for IS1655 from Neisseria meningitis<ref><nowiki><pubmed>17367392</pubmed></nowiki></ref> (Fig. IS30.5). Another IS30 family member, ISCg2 from Corynebacterium glutamicum, has also been shown to insert into a relatively well defined sequence which exhibits some palindromic symmetry<ref><nowiki><pubmed>10589846</pubmed></nowiki></ref> while yet another, IS1630 from Mycoplasma fermentans, appears to show a preference for rather long target with extensive palindromic sequences<ref><nowiki><pubmed>10601219</pubmed></nowiki></ref>. | Like IS21<ref><nowiki><pubmed>3034717</pubmed></nowiki></ref> and IS911<ref><nowiki><pubmed>10760133</pubmed></nowiki></ref>, IS30 shows a preference for insertion next to nucleotide sequences resembling its ends<ref><nowiki><pubmed>9393723</pubmed></nowiki></ref>. Furthermore, this region has been delimited approximately to the Tpase binding site. In addition, preferential insertion into a site in bacteriophage l (LHS) and P1 (OHS) has been observed both in E.coli<ref><nowiki><pubmed>8389976</pubmed></nowiki></ref> and Salmonella<ref><nowiki><pubmed>10570999</pubmed></nowiki></ref>. These sequences are somewhat degenerate palindromes characterized by a 24 bp symmetric consensus<ref><nowiki><pubmed>9643538</pubmed></nowiki></ref> (Fig. IS30.4). The sequence relationship between these various hotspots has yet to be explored. A similar type of insertion specificity was observed for IS1655 from Neisseria meningitis<ref><nowiki><pubmed>17367392</pubmed></nowiki></ref> (Fig. IS30.5). Another IS30 family member, ISCg2 from Corynebacterium glutamicum, has also been shown to insert into a relatively well defined sequence which exhibits some palindromic symmetry<ref><nowiki><pubmed>10589846</pubmed></nowiki></ref> while yet another, IS1630 from Mycoplasma fermentans, appears to show a preference for rather long target with extensive palindromic sequences<ref><nowiki><pubmed>10601219</pubmed></nowiki></ref>. | ||
[[Image:FIg. IS30.4.png|thumb|center|850px|Fig. IS30.4]] | [[Image:FIg. IS30.4.png|thumb|center|850px|Fig. IS30.4]] | ||
| − | [[Image:FIg. IS30.5.png|thumb|center|850px|Fig. IS30.5]] | + | [[Image:FIg. IS30.5.png|thumb|center|850px|Fig. IS30.5 Consensus of 110 IS''1655'' target sequences comprising: 38 IS1655 insertions in public databases; 72 target sites of IS1655 ref copy isolated from different plasmids and the E. coli chromosome. The target sites were generated by removing the entire IS1655 copy and one of the 3 bp direct repeats bracketing the element in the original sequence. The sequences were oriented according to the orientation of the IS1655 copy. Originally the left half of each target sequence flanked the IRL and the right half flanked the IRR of the element. Conserved bases were not found out of the �9 bp region. Sequence logo was generated for the central 27 bp of target sites by the WebLogo server (<nowiki>http://weblogo.berkeley.edu/</nowiki>).]] |
====ISApl1==== | ====ISApl1==== | ||
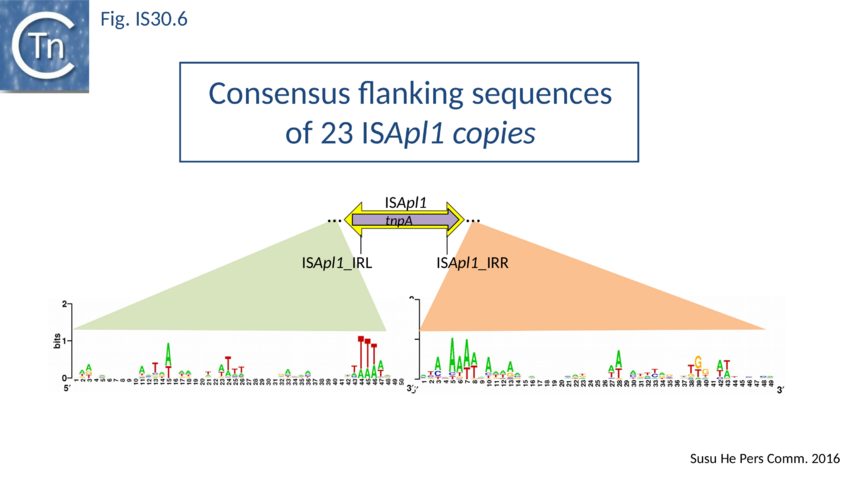
ISApl1 plays an important role in transmission of resistance to the last resort antibiotic, colistin. Liu<ref><nowiki><pubmed>26711359</pubmed></nowiki></ref><ref><nowiki><pubmed>26603172</pubmed></nowiki></ref><ref><nowiki><pubmed>26973307</pubmed></nowiki></ref><ref><nowiki><pubmed>27324774</pubmed></nowiki></ref><ref><nowiki><pubmed>27090630</pubmed></nowiki></ref><ref><nowiki><pubmed>27322919</pubmed></nowiki></ref><ref><nowiki><pubmed>27230792</pubmed></nowiki></ref>. It was originally identified in Actinobacillus pleuropneumoniae from pigs<ref><nowiki><pubmed>18065168</pubmed></nowiki></ref> and has typical IS30 family characteristics: 1070 bp long with a 924 bp transposase orf and flanked by 27 bp inverted repeats. Four copies occurred in the original AP76 strain, one of which interrupts the apxIVA toxin gene. It was also reported to be present in certain field isolates but not in the type strain. All four insertions generate a two base pair DR and occur in an AT rich region with some palindrome-like characteristics and a central GC rich region (see Fig. IS30.6) similar to that observed for IS1665<ref><nowiki><pubmed>17367392</pubmed></nowiki></ref>. Its transposase is very similar to that of IS30, especially around the active site. | ISApl1 plays an important role in transmission of resistance to the last resort antibiotic, colistin. Liu<ref><nowiki><pubmed>26711359</pubmed></nowiki></ref><ref><nowiki><pubmed>26603172</pubmed></nowiki></ref><ref><nowiki><pubmed>26973307</pubmed></nowiki></ref><ref><nowiki><pubmed>27324774</pubmed></nowiki></ref><ref><nowiki><pubmed>27090630</pubmed></nowiki></ref><ref><nowiki><pubmed>27322919</pubmed></nowiki></ref><ref><nowiki><pubmed>27230792</pubmed></nowiki></ref>. It was originally identified in Actinobacillus pleuropneumoniae from pigs<ref><nowiki><pubmed>18065168</pubmed></nowiki></ref> and has typical IS30 family characteristics: 1070 bp long with a 924 bp transposase orf and flanked by 27 bp inverted repeats. Four copies occurred in the original AP76 strain, one of which interrupts the apxIVA toxin gene. It was also reported to be present in certain field isolates but not in the type strain. All four insertions generate a two base pair DR and occur in an AT rich region with some palindrome-like characteristics and a central GC rich region (see Fig. IS30.6) similar to that observed for IS1665<ref><nowiki><pubmed>17367392</pubmed></nowiki></ref>. Its transposase is very similar to that of IS30, especially around the active site. | ||
| − | [[Image:FIg. IS30.6.png|thumb|center|850px|Fig. IS30.6]] | + | [[Image:FIg. IS30.6.png|thumb|center|850px|Fig. IS30.6 Weblogo of sequences flanking 23 ISApl1 copies.]] |
PCR analysis demonstrated the presence of a junction fragment in which IRL and IRR are separated by a GG dinucleotide. This is the DR observed at the ISApl1 insertion site in A. pleuropneumoniae downstream of a bla gene but is not present at the other three insertion sites in this strain. The presence of this junction fragment suggests that ISApl1, like IS30 and IS1665, occurs using a circular DNA intermediate during transposition. | PCR analysis demonstrated the presence of a junction fragment in which IRL and IRR are separated by a GG dinucleotide. This is the DR observed at the ISApl1 insertion site in A. pleuropneumoniae downstream of a bla gene but is not present at the other three insertion sites in this strain. The presence of this junction fragment suggests that ISApl1, like IS30 and IS1665, occurs using a circular DNA intermediate during transposition. | ||
Revision as of 15:19, 8 May 2020
Contents
General features and Original Identification
IS30 was identified as an insertion into the phage P1 genome[1][2].
Distribution
The IS30 family currently comprises more than 100 members from 94 members from nearly 80 bacterial species and an example, ISC1041, has also been found in the Archaea[3]. IS30-like Tpases have also been found as integral parts of certain Integrative Conjugative Elements (ICE) from Staphylococcus aureus (MRSA)[4]. An open reading frame found originally in a Spiroplasma citri phage (Sc1) but since identified also in the Spiroplasma citri genome is distantly related to the family.
Organization
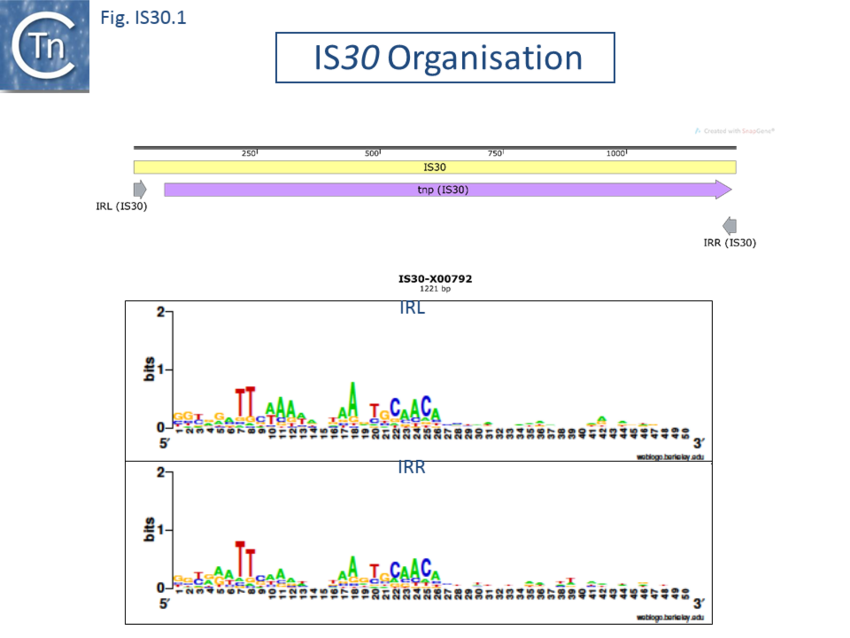
IS30 family members have lengths of between 1027 bp (IS1070) and 1636 bp (ISCg2) with a single orf of between 293 and 398 codons spanning almost the entire length. The termination codon is generally very close to the right end. Alignment of the putative Tpases reveals a well conserved DD(33)E motif.
Family members can activate neighboring genes by creation of a hybrid promoter on insertion next to a -10 promoter element[5][6][7][8].
The Tpases show several well-conserved regions. One, in the N-terminus includes a potential helix–turn–helix (HTH) motif, which, in the case of IS30, is responsible for IR binding[9][10]. Another in the C-terminal region contains the DD(33)E motif. (Fig. IS30.1)
The terminal IRs are in the range of 20-30 bp and exhibit significant conserved sequence signatures (Fig. IS30.1). The tips of the elements, as defined by the various authors, show significant sequence variation, although in most elements the IR have not been experimentally confirmed. Where it has been determined, 2 bp or 3 bp direct target repeats are generated on insertion, but there are several exceptions in which the DR is between 12 and 32 bp.[e.g. IS1630, IS1470, IS658, ISApl1, ISL7, ISLpl1[11]]. One member of this family, IS1630 (1377bp) from Mycoplasma fermentans is flanked by long but variable DRs of between 19 and 26bp[12].
Presence in Compound Transposons
IS30 is known to function in a compound transposon structure[13] and various IS30 family members also have been identified as part of composite transposons. In particular, Tn6330 which is composed of two copies of ISApl1 flanking the colistin resistance gene, mcr-1[14][15][16].
IS30
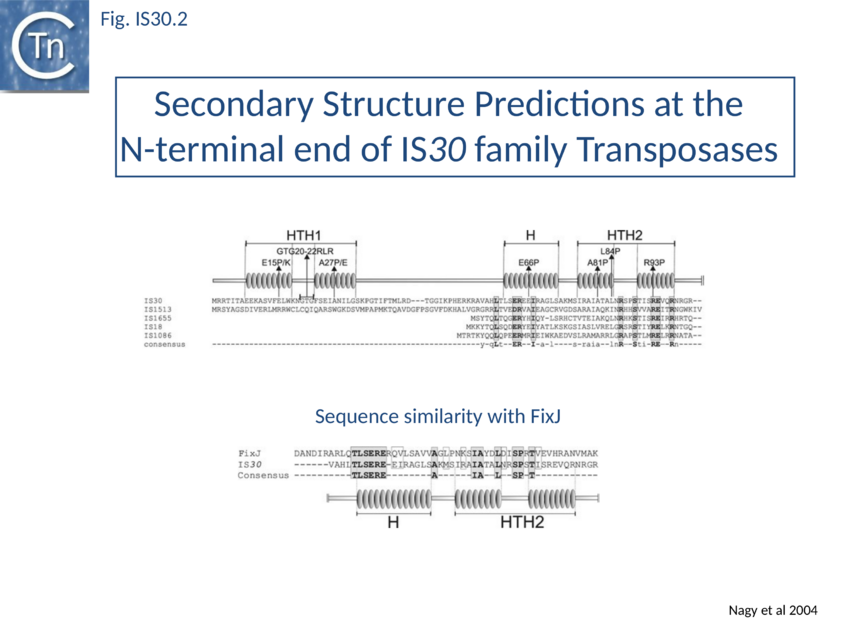
Of the different members of this family, IS30 is the best characterised. It is 1221 bp long with 26 bp terminal IRs [16] and mediates several types of DNA rearrangements including simple insertion, cointegrate formation, deletion and inversion[17][18][19]. It shows pronounced insertion specificity and generates DRs of 2 bp [1]. It terminates with the dinucleotide 5'-CA-3'[20] [16] although many other members of the family do not. The single long orf is preceded by a relatively weak promoter (p30A) capable of driving Tpase expression with its -35 hexamer within IRL[21] and contains a weak internal transcription terminator. The transposase is predicted to include two N-terminal HTH motifs (Fig. IS30.2) and show significant homology to FixJ, a Sinorhizobium meliloti transcriptional regulator[22]. Premature transcription termination at this site could lead to the formation of Tpase molecules truncated at their C-terminal end carrying the catalytic DDE motif. Since the specific DNA binding domain which recognises the terminal IRs is located in the N-terminal third of the protein[23], the potential truncated derivatives may lack the catalytic domain and regulate transposition activity by competition or interaction with a full length Tpase. This arrangement may play the same role at the transcriptional level as does frameshifting at the translational level for certain other elements.
A second weak promoter (p30C) located on the opposite strand upstream of a short orf has been detected[24] and it has recently been shown that a small 150 nucleotide transcript driven from this promoter and contained entirely within the Tpase transcript acts as an antisense control by reducing translation of the Tpase mRNA[25].
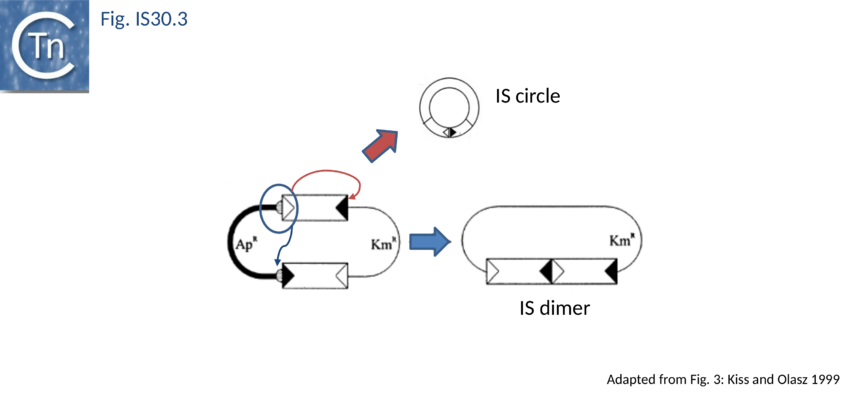
The founding member of the family, IS30, is also the best characterized at the mechanistic level[26][27][28][29][30][31][32][33][34] and an in vitro transposition system has been developed[35]. This 1221 bp long Escherichia coli element belongs to a growing class of ISs known to transpose through an intermediate formed by abutting the IRs, Donor Primed Transposon Replication. Here the IR are separated by 2 bp[36][37][38]. Such an IR junction can be created by formation of a dimer of two directly repeated IS copies or by the formation of transposon circles. Both IS minicircles and dimers have been observed. IR–IR junctions have also been detected in some other IS30 family members such as IS18[39], IS4351[40] and IS1470[41]. A structure in which two IS30 ends are linked by a single strand bridge (forming a figure of eight structure on a circular plasmid), has been identified[42].
As in the case of IS21 and IS3 families, the assembly of abutted IRL and IRR copies creates a strong promoter. In a tandem dimer of the element, this may result in increased expression of the Tpase from the downstream copy[43]. The IRL-IRR junction is also recombinationally unstable. It promotes transposition reactions at high frequency in vivo[44] presumably in a similar way to the equivalent junction of IS21. The activity of the junction appears maximal when the spacer between the abutted ends is 2 bp (the length of target duplications introduced when IS30 inserts). Lower activities are obtained with 1 and 3 bp spacers while little or no activity is observed for 0 bp or >3bp (T. Naas and W. Arber, pers. comm.). Strong evidence has recently been presented indicating that donor plasmid dimerisation is followed by interaction of one appropriately oriented end from each IS copy to form an abutted IS intermediate (see Fig. IS30.3). The IS junction in this intermediate is highly active in transposition and plays an important role in transposition of IS30[45].
Like IS21[46] and IS911[47], IS30 shows a preference for insertion next to nucleotide sequences resembling its ends[48]. Furthermore, this region has been delimited approximately to the Tpase binding site. In addition, preferential insertion into a site in bacteriophage l (LHS) and P1 (OHS) has been observed both in E.coli[49] and Salmonella[50]. These sequences are somewhat degenerate palindromes characterized by a 24 bp symmetric consensus[51] (Fig. IS30.4). The sequence relationship between these various hotspots has yet to be explored. A similar type of insertion specificity was observed for IS1655 from Neisseria meningitis[52] (Fig. IS30.5). Another IS30 family member, ISCg2 from Corynebacterium glutamicum, has also been shown to insert into a relatively well defined sequence which exhibits some palindromic symmetry[53] while yet another, IS1630 from Mycoplasma fermentans, appears to show a preference for rather long target with extensive palindromic sequences[54].
ISApl1
ISApl1 plays an important role in transmission of resistance to the last resort antibiotic, colistin. Liu[55][56][57][58][59][60][61]. It was originally identified in Actinobacillus pleuropneumoniae from pigs[62] and has typical IS30 family characteristics: 1070 bp long with a 924 bp transposase orf and flanked by 27 bp inverted repeats. Four copies occurred in the original AP76 strain, one of which interrupts the apxIVA toxin gene. It was also reported to be present in certain field isolates but not in the type strain. All four insertions generate a two base pair DR and occur in an AT rich region with some palindrome-like characteristics and a central GC rich region (see Fig. IS30.6) similar to that observed for IS1665[63]. Its transposase is very similar to that of IS30, especially around the active site.
PCR analysis demonstrated the presence of a junction fragment in which IRL and IRR are separated by a GG dinucleotide. This is the DR observed at the ISApl1 insertion site in A. pleuropneumoniae downstream of a bla gene but is not present at the other three insertion sites in this strain. The presence of this junction fragment suggests that ISApl1, like IS30 and IS1665, occurs using a circular DNA intermediate during transposition.
Following the initial report in 2016 of resistance to a last resort antibiotic, the polymyxin colistin, by a transmissible plasmid-associated mcr-1 gene[64], it was rapidly recognised (within the year) that the gene, which encodes a Phosphoethanolamine Transferase[65], was present in Enterobacteriaceae from five continents: Asia, Europe, Africa, South America, and North America (reviewed in [66]). Based on the analysis of 77mcr-1-containing sequences (of which 19 were too fragmented to be useful) representing every unique sequence in NCBI as of August 2016, a common 2,607-bp DNA segment was identified that in many cases is flanked on one or both ends by ISApl1[67] [14]. This fragment included the mcr-1 gene together with a truncated copy of a gene called pap2. This analysis led to the proposal that mcr-1 was transmitted by an ISApl1 composite transposon, later called Tn6330[68] which has, in some cases, subsequently lost one or both ISApl1 copies. Analysis of Tn6330 transposition has identified the expected transposition intermediate[69]: a transposon circle which carries a pair of abutted IS copies (as shown for IS30[70]; Fig. IS30.3). A second structure found in the study, a circular form with only a single IS copy may be equivalent to one of the transposition related deletion products described for IS30[71] or simply by homologous recombination between the pair of directly repeated ISApl1. This was also observed in another study[72].
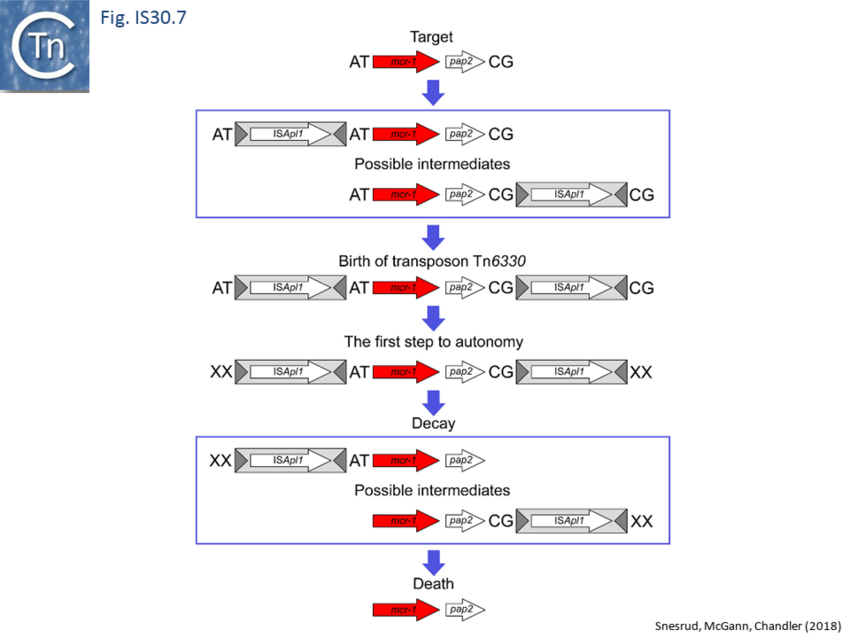
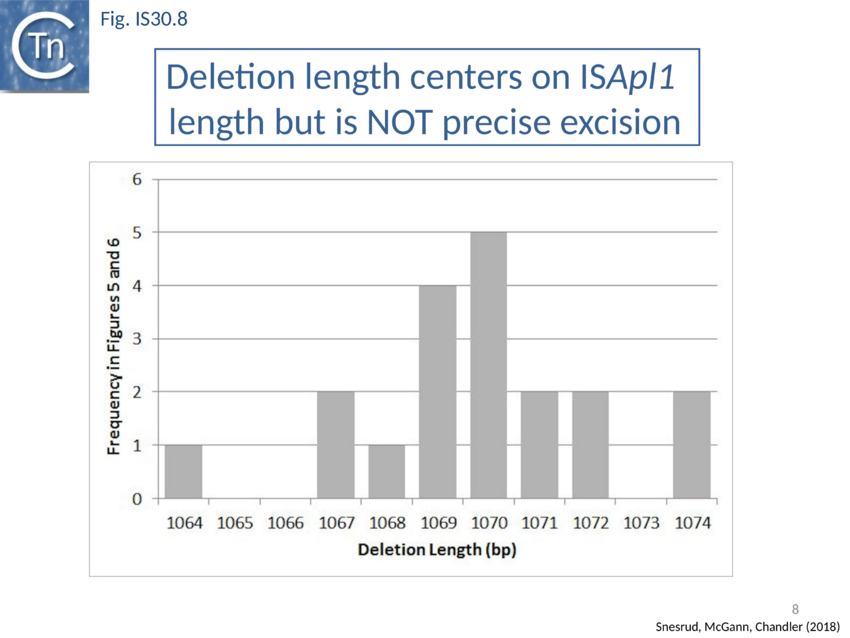
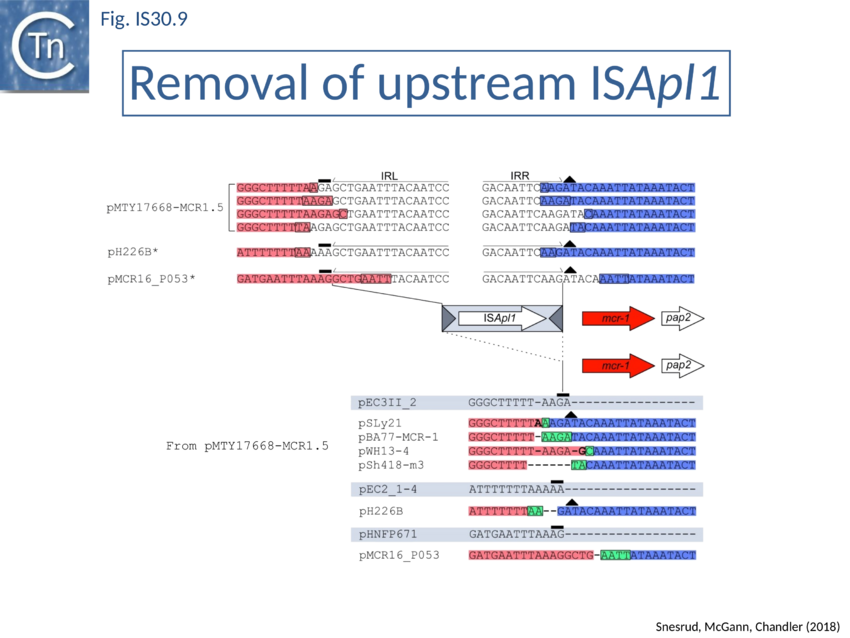
Further analysis with an enlarged database[73] provided further support for this and resulted in a model for the mobilisation of mcr-1 from the chromosome of a species closely resembling Moraxella sp. MSG13-C03 by insertion of ISApl1 copies upstream and downstream (Fig. IS30.7), transmission by transposition to a target plasmid followed by interbacterial transfer and acquisition by additional conjugative plasmids. The sites of insertion were as expected for ISApl1 insertion sites (Fig. IS30.6), an observation which has been extensively confirmed[74]. The full transposon was then then proposed to decay by deletion of the downstream and/or upstream ISApl1 copies leaving Tn6330 derivatives carrying a single downstream or upstream IS copy or devoid of both IS copies (Fig. IS30.7). Based on the transposition mechanism of IS30 family members, and on the observation that, although the deletion endpoints were variable from case to case, the length of the deletion was remarkably constant (Fig. IS30.8) representing the exact IS length, it was further proposed that IS deletion occurred by frequent abortive IS transposition (Fig. IS30.9). IS30 family transposition appears to occur by a copy-out-paste-in mechanism which involves replication (Fig. IS30.9 left). In the deletion model, strand-switching between micro homologies during replication step (Fig. IS30.9 right) would lead to the “looping out” of the IS rather than replication through to the opposite end. This could lead to removal of the IS copy during further plasmid replication[75]. This model is supported by data obtained several decades ago in which similar loss of IS30 was shown to occur with relatively high frequency and involve microhomologies[76]. Unfortunately, a more recent study which analysed the stability of Tn6330 did not directly address the loss of flanking ISApl1 or the necessity of transposition enzymes or intact terminal IRs[77].
Bibliography
- ↑ <pubmed>6090868</pubmed>
- ↑ <pubmed>385224</pubmed>
- ↑ <pubmed>9654137</pubmed>
- ↑ <pubmed>19648240</pubmed>
- ↑ <pubmed>3039299</pubmed>
- ↑ <pubmed>3038844</pubmed>
- ↑ <pubmed>9756793</pubmed>
- ↑ <pubmed>23158541</pubmed>
- ↑ <pubmed>2154486</pubmed>
- ↑ <pubmed>15469518</pubmed>
- ↑ <pubmed>15469518</pubmed>
- ↑ <pubmed>10601219</pubmed>
- ↑ <pubmed>2546856</pubmed>
- ↑ <pubmed>29440577</pubmed>
- ↑ <pubmed>27620479</pubmed>
- ↑ <pubmed>30927434</pubmed>
- ↑ <pubmed>385224</pubmed>
- ↑ <pubmed>8389976</pubmed>
- ↑ <pubmed>7911771</pubmed>
- ↑ <pubmed></pubmed>
- ↑ <pubmed>2414100</pubmed>
- ↑ <pubmed>15469518</pubmed>
- ↑ <pubmed>2154486</pubmed>
- ↑ <pubmed>2414100</pubmed>
- ↑ <pubmed>9461341</pubmed>
- ↑ <pubmed>2154486</pubmed>
- ↑ <pubmed>2154486</pubmed>
- ↑ <pubmed>8389976</pubmed>
- ↑ <pubmed>9303555</pubmed>
- ↑ <pubmed>9393723</pubmed>
- ↑ <pubmed>10540284</pubmed>
- ↑ <pubmed>14665688</pubmed>
- ↑ <pubmed>12724391</pubmed>
- ↑ <pubmed>17367392</pubmed>
- ↑ <pubmed>18022196</pubmed>
- ↑ <pubmed>3039299</pubmed>
- ↑ <pubmed>8389976</pubmed>
- ↑ <pubmed>10540284</pubmed>
- ↑ <pubmed>9756793</pubmed>
- ↑ <pubmed>2826402</pubmed>
- ↑ <pubmed>9919679</pubmed>
- ↑ <pubmed>18022196</pubmed>
- ↑ <pubmed>3039299</pubmed>
- ↑ <pubmed>8389976</pubmed>
- ↑ <pubmed>10540284</pubmed>
- ↑ <pubmed>3034717</pubmed>
- ↑ <pubmed>10760133</pubmed>
- ↑ <pubmed>9393723</pubmed>
- ↑ <pubmed>8389976</pubmed>
- ↑ <pubmed>10570999</pubmed>
- ↑ <pubmed>9643538</pubmed>
- ↑ <pubmed>17367392</pubmed>
- ↑ <pubmed>10589846</pubmed>
- ↑ <pubmed>10601219</pubmed>
- ↑ <pubmed>26711359</pubmed>
- ↑ <pubmed>26603172</pubmed>
- ↑ <pubmed>26973307</pubmed>
- ↑ <pubmed>27324774</pubmed>
- ↑ <pubmed>27090630</pubmed>
- ↑ <pubmed>27322919</pubmed>
- ↑ <pubmed>27230792</pubmed>
- ↑ <pubmed>18065168</pubmed>
- ↑ <pubmed>17367392</pubmed>
- ↑ <pubmed>26603172</pubmed>
- ↑ <pubmed>26603172</pubmed>
- ↑ <pubmed>27342545</pubmed>
- ↑ <pubmed>27620479</pubmed>
- ↑ <pubmed>30927434</pubmed>
- ↑ <pubmed>30723461</pubmed>
- ↑ <pubmed>10540284</pubmed>
- ↑ <pubmed>10540284</pubmed>
- ↑ <pubmed>30927434</pubmed>
- ↑ <pubmed>29440577</pubmed>
- ↑ <pubmed>30723461</pubmed>
- ↑ <pubmed>29440577</pubmed>
- ↑ <pubmed>10545262</pubmed>
- ↑ <pubmed>30927434</pubmed>